- Home Page
- Company Profile
-
Our Products
- Anti Diabetic Tablet
- Gliclazide Tablets
- Atorvastatin Calcium Tablet
- Alpha Lipoic Acid Capsules
- Voglibose Tablets
- Vildagliptin and Metformin Tablets
- Voglibose Tablets
- Pioglitazone Tablets
- Metformin Hydrochloride Tablets
- Metformin Hydrochloride Tablets
- Metformin Hydrochloride Tablets
- Canagliflozin Tablet
- Alogliptin Metformin Tablets
- Glimepiride, Metformin And Pioglitazone Tablet
- Metformin SR Tablets
- Voglibose Mouth Dissolving Tablets
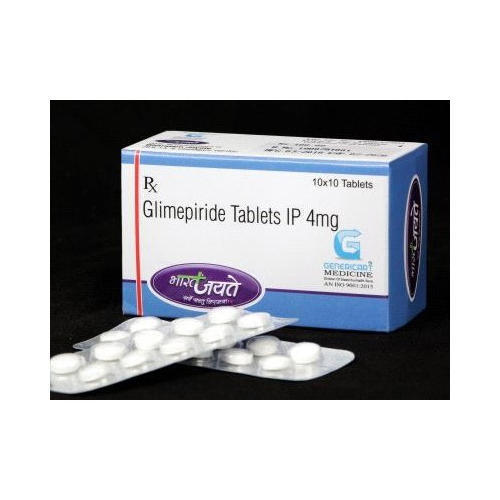
- Glimepride Tablets
- Teneligliptin Tablet
- Tripride Tablets
- Pioglitazone Tablet
- Gliclazide Tablets
- Glimepride Metformin Tablets
- Pioglitazone hydrochloride and Extended release metformin hydrochloride tablets
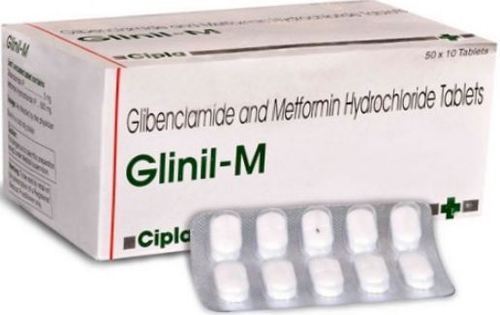
- Metformin Hydrochloride Sustained Release and Glibenclamide Tablets
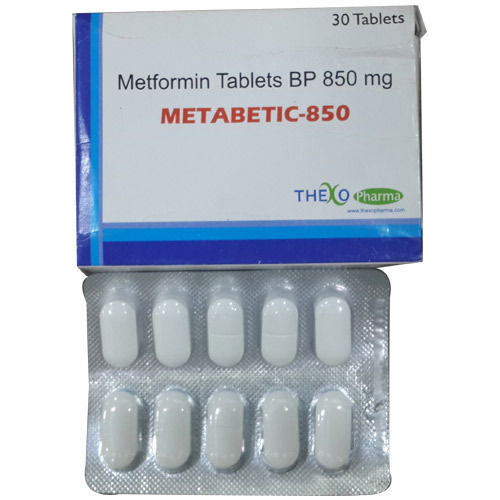
- Metformin Tablets
- Pioglitazone Hydrochloride Tablets
- Vildagliptin and Metformin Tablets
- Vildagliptin and Metformin Tablets
- Vildagliptin Tablets
- Glimepiride Tablets
- Metformin Tablets
- Metformin Tablets
- Repagliptine Tablets
- Glimepiride Tablets
- Metformin Hydrochloride Sustained release and Glimepride Tablets
- Saxagliptin Tablets
- Saxagliptin Tablets
- Metformin Hydrochloride And Glime Pride Tablets
- Canagliflozin Tablets
- Pioglitazone Tablets
- Empagliflozin tablet
- Glimepiride Tablets
- Empagliflozin TabletS
- Gliclazide Tablets
- Glimepiride Tablets
- Gliclazide Tablets
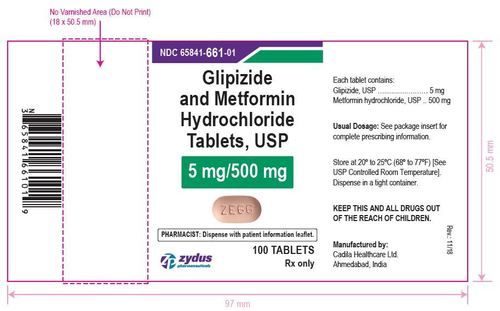
- Glipizide Tablets
- Glipizide and Metformin Hydrochloride Tablets
- Glipizide and Metformin Hydrochloride Tablets
- Voglibose and Metformin HCL tablets
- Voglibose Mouth Dissolving Tablets
- Anti Fungal Tablet
- Fluconazole Tablets
- Combi Kit of Esomeprazole, Clarithromycin and Tinidazole
- Miconazole Nitrate Capsules
- Voriconazole Tablets
- Voriconazole Tablets
- Nystatin Tablets
- Nystatin Tablets
- Ketoconazole Tablets
- Fluconazole Tablets
- Aluminium Phosphide Tablets Alp
- Voriconazole Tablets
- Itraconazole Capsules
- Amphotericin B Emulsion
- Griseofulvin Tablets
- Ampicillin Capsule
- Fluconazole Capsules
- Ketoconazole Tablets
- Itraconazole Capsules
- Vildagliptin and Metformin Tablets
- Ivermectin Tablets
- Ivermectin Tablets
- Clindamycin Clotrimazole Tinidazole soft Gelatin Capsule
- Fluconozole Tablets
- Griseofulvin Tablets
- Fluconozole Tablets
- Griseofulvin Tablets
- Clotrimazole Vaginal Tablets
- Calcium Carbonate Chewable Tablets
- Clotrimazole Vaginal Tablets
- Fluconazole Tablets
- Fluconazole Capsules
- Fluconazole, Azithromycin & Secnidazole Combi Kit
- Combi Kit of Lansoprazole, Clarithromycin And Tinidazole
- Fluconazole Tablets
- Fluconazole Capsules
- Fluconazole Capsules
- Ketoconazole Tablets
- Combi Kit of Omeprazole, Clarithromycin and Tinidazole
- Azithromycin Fluconazole and Ornidazole Kits
- Anti Hiv Tablet
- Zidovudine Tablets
- Viraday Tablet
- Darunavir Tablets
- Abacavir Lamivudine Zidovudine Tablets Trizivir
- Lamivudine Zidovudine & Nevirapine Tablets
- Efavirenz Tablets
- Dolutegravir Tablets
- Lamivudine Zidovudine Tablet
- Tenofovir Tablets
- Abacavir lamivudine Tablets
- Sofosbuvir Tablets
- Tenofovir Efavirenz Tablet
- Lamivudine and Zidovudine Tablets
- Anti Malarial Product
- Amodiaquine Hydrochloride Tablets
- Chloroquine Phosphate Tablet
- Sulphadoxin Pyrimethamine Tablet
- Chloroquine Phosphate Tablet
- Arteether Injection
- Artesunate For Injection
- Artesunate For Injection
- Arteether Injection
- Artesunate For Injection
- Arteether Injection
- Artemether & Lumefantrine Powder
- Amodiaquine Oral Suspension
- Chloroquine Phosphate Syrup
- Dihydroartemisinin and Piperaquine Phosphate For Oral Suspension
- Sulphadoxin + Pyrimethamine Tablet
- Artemether Capsule
- Primaquine Tablets
- Primaquine Tablets
- Artesunate And Mefloquin Tablets
- Artesunate Amodiaquine Tablets
- Quinine Sulphate Tablets
- Quinine Sulphate Tablets
- L-Ornithine L-Aspartate Sachets
- Artemether injection
- Artemether Lumefantrine Tablets
- Primaquine Phosphate Tablet
- Atovaquone Tablet
- Antimalarial Kit
- Artesunate Injection
- Artesunate Tablets
- Chloroquine Phosphate Tablets
- Quinine Sulphate Tablets
- Artemisinin and Piperaquine Tablets
- Artemether and Lumefantrine powder for oral suspension
- Artesunate & Mefloquine Tablets Kit
- Quinine Dihydrochloride Injection
- Quinine Dihydrochloride Injection
- Quinine Dihydrochloride Injection
- Quinine Dihydrochloride Injection
- Artesunate Tablets
- Arteether + Lumefantrine Tablets
- Mefloquine Hydrochloride Tablets
- Chloroquine Phosphate Tablet
- Chloroquine Phosphate Tablet
- Artesunate and Sulphadoxine & Pyrimethamine Tablets
- Arteether + Lumefantrine Tablets
- Arteether + Lumefantrine Tablets
- Artesunate Tablets
- Artesunate + Artesunate tablets
- Artesunate + Amodiaquine tablets
- Artemether & Lumefantrine Oral Suspension
- Amodiaquine Hydrochloride Tablets
- Amodiaquine Hydrochloride Tablets
- Artemether Injection
- Anti Spasmodic Tablet
- Cefadroxil for Oral Suspension
- Cetrizine Tablets
- Hyoscine butylbromide Tablets
- Antipyretic Medicines
- Deflazacort Tablets
- Sulfasalazine Tablets
- Etoricoxib Tablet
- Erythromycin Stearate Tablets
- Aceclofenac Serrtiopeptidase Paracetamol Tablets
- Diclofenac Serratiopeptidase Tablets
- Analgin Tablets
- Frusemide, Amiloride Tablets
- Nutraceutical Product
- Psyllium Husk Effervescent Powder
- Gamma Banzene Hexachloride Lotion
- Benzoyl Peroxide Lotion
- Betamethasone and Salicylic Acid Lotion
- Betamethasone Valerate Lotion
- Dimethicone Lotion
- Fluocinolone Acetonide Lotion
- Cefpodoxime Proxetil for Oral Suspension
- Clindamycin Phosphate Lotion
- Clotrimazole Mouth Paint
- Benzydamine Mouthwash
- Activated Charcoal Powder
- Benzoyl Peroxide Lotion
- Activated Charcoal Powder
- Amoxicillin and Clavulanate Potassium for Oral Suspension
- Calamine Lotion
- Alphacalcidol with Calcium Tablets
- Dipirona Gotas Drops
- Vitamin Supplement
- Whey Protein Powder For Muscles Growth & Sports
- Zinc Oxide Powder
- Turpentine Liniment Oil
- Trimethoprim and Sulfamethoxazole Oral suspension
- Povidone Iodine Powder
- Sorbitol Oral Powder
- Clotrimazole Absorbent Dusting Powder
- Vigabatrin Oral Powder
- Neomycin and Bacitracin Powder
- Liquid Paraffin
- L-Arginine + Proanthocyanidin Sachet
- Magnesium Sulphate paste
- Ofloxacin Suspension
- Loratadine Oral Solution
- Levocetirizine Dihydrochloride Syrup
- Ondansetron Solution
- Hydrogen Peroxide Solution
- Benzyl Benzoate
- Desonide Lotion
- Multivitamin With Minerals Tablets
- MULTIVITAMIN TABLET
- Cefadroxil Tablets
- Neomycin and Bacitracin with Sulfacetamide Powder
- Biotin Capsule
- Potassium Iodide Tablets
- Pharmaceutical Syrup
- Nystatin for Oral Suspension
- Aluminium and Magnesium Hydroxide Oral Suspension
- Lactulose solution Syrup
- Captopril Oral Solution
- Cod-liver Oil Emulsion syrup
- Deltamethrin Solution
- Flucloxacillin Granules for Oral Solution
- Haloperidol Oral Solution
- Hydroxyzine Hydrochloride Syrup
- Ondansetron Solution
- Quinine Sulphate Suspension
- Montelukast and Levocetirizine Oral Syrup
- Lamivudine Oral Solution
- Chlorpheniramine Maleate & Paracetamol Oral Suspension
- Albendazole and Ivermectin Suspension
- Belladonna Tincture
- Cetirizine Hydrochloride Syrup
- Levetiracetam Solution
- Iron + Folic Acid + Vit. B Syrup
- Thioridazine Hcl Tablets
- Zidovudine Oral Solution
- Nevirapine Oral Suspension
- Cholecalciferol Drops
- Promethazine Hydrochloride Oral Solution
- Zinc Sulfate Syrup
- Ofloxacin Oral Suspension
- Primidone Suspension
- Theophylline & Etofylline Syrup
- Acriflavin+Glycerin Solution
- Carbocisteine Syrup
- Ampicillin for Oral Suspension
- Povidone Iodine Solution
- Amoxicillin and Potassium Clavulanate for Oral Suspension
- Amoxicillin and Clavulanate Potassium Powder
- Paracetamol Oral Suspension
- Metronidazole Oral Suspension
- Bromhexine Syrup
- Amoxicillin and Clavulanate Potassium for Oral Suspension
- Carbocisteine Syrup
- Sucralfate and Oxetacaine Suspension
- Desloratadine Oral Solution
- Amoxicillin + Potassium Clavulanate for Oral Suspension
- Albendazole Oral Suspension
- Ambroxol Hydrochloride + Cetirizine Hydrochloride Syrup
- Cefaclor oral suspension
- Bromhexine Hydrochloride Syrup
- Cefadroxil Drops
- Ambroxol Hydrochloride Syrup
- Azithromycin Syrup
- Paediatric Digoxin Oral Solution
- Carbocisteine Syrup
- Amoxicillin and Clavulanate Potassium for Oral Suspension
- Aluminium and Magnesium Hydroxide Suspension
- Phenoxymethylpenicillin Oral Solution
- Acriflavine lotion Syrup
- Captopril Oral Solution
- Acetaminophen Oral Solution
- Amoxicillin for Oral Suspension
- Povidone-Iodine + Metronidazole Solution
- Albendazole Oral Suspension
- Isoniazid Syrup
- Phenytoin Oral Suspension
- Chlorpromazine Hydrochloride Syrup
- Dicloxacillin Sodium For Oral Suspension
- Efavirenz Oral Solution
- Deferiprone Solution
- Fluconazole for Oral Suspension
- Griseofulvin for oral Suspension
- Domperidone Oral Suspension
- Cyproheptadine Hcl + Tricholine citrate + Sorbitol Syrup
- Cloxacillin Sodium for Oral Solution
- Cefalexin Oral Suspension
- Ferrous Fumarate Syrup
- Dextromethorphan Syrup
- Ciprofloxacin Oral Suspension
- Erythromycin EthylSuccinate For Oral Suspension
- Cephalexin for Oral Suspension
- Cefalexin Oral Drops
- Fosamprenavir Calcium Oral Suspension
- Dicyclomine Hcl & Activated Dimethicone Suspension
- Ferric Ammonium Citrate, Folic Acid , Vitamin B12 Syrup
- Cefpodoxime and Clavulanic Oral Suspension
- Cefixime For Oral Suspension
- Diphenhydramine Hcl + Ammonium Chloride + Sodium Citrate Syrup
- Disodium Hydrogen Citrate Liquid
- Domperidone Suspension
- Chlorpheniramine Maleate Oral Solution
- Ferrous Ascorbate + Folic Acid Syrup
- Ferrous Fumarate Oral Suspension
- Diclofenac Potassium Oral Suspension
- Chlorpheniramine Maleate Oral Solution
- Clotrimazole Topical Solution
- Clindamycin Palmitate Hydrochloride for Oral Solution
- Erythromycin Estolate for Oral Suspension
- Erythromycin Ethyl Succinate For Oral Suspension
- Diphenhydramine Oral Solution
- Chlorpheniramine Maleate Oral Solution
- Desloratadine Syrup
- Dexchlorpheniramine Maleate Syrup
- Fexofenadine Hcl Syrup
- Erythromycin Estolate for Oral Suspension
- Erythromycin Stearate for Oral Suspension
- Cefpodoxime Proxetil For Oral Suspension
- Fluconazole for Oral Suspension
- Cloxacillin Sodium for Oral Solution
- Citicoline Oral Solution
- Dexamethasone Elixir
- Dexamethasone Oral Solution
- Dexamethasone Oral Solution
- Dicyclomine Hydrochloride Oral Solution
- Cloxacillin Sodium Oral suspension
- Anti Tussive Anti Allergic Decongestant Syrup
- Cyproheptadine HCl with B-Complex Syrup
- Flucloxacillin Granules for Oral Solution
- Cholestyramine for oral Suspension
- Furazolidone Oral Suspension
- Chlorpheniramine Maleate Oral Solution
- Cefpodoxime for Oral Suspension
- Diphenhydramine Syrup
- Cefdinir for oral suspension
- Ceftibuten for Suspension
- Cetrizine hydrochloride Syrup
- Clindamycin Solution
- Cyproheptadine Hydrochloride Syrup
- Ferric Ammonium Citrate + Folic Acid + Vitamin B12 Oral Suspension
- Clarithromycin Oral Suspension
- Chloral Hydrate Syrup
- Cefuroxime Axetil for Oral Suspension
- Cefixime & Potassium Clavulanate Oral Suspension
- Disodium Hydrogen Citrate Syrup
- Cefdinir for oral suspension
- Cefuroxime Axetil for Oral Suspension
- Dimetindene Maleate Drops
- Cholestyramine Oral Suspension
- Ferrous Sulphate and Folic Acid Syrup
- Calcium Carbonate with Vitamin D3 Suspension
- Amoxicillin Sachet Powder
- Dicyclomine Hydrochloride Syrup
- Amoxicillin and Bromhexine for oral suspension
- Albendazole Powder
- Aluminium and Magnesium Hydroxide and Dimethicone Suspension
- Ambroxol Hydrochloride + Cetirizine Hydrochloride Syrup
- Calcium Carbonate Oral Suspension
- Ferric Ammonium Citrate + Folic Acid + Vitamin B12 Syrup
- Abacavir Oral Solution
- Calcium Carbonate with Vitamin D3 Suspension
- Aqueous Iodine and Potassium Iodide Solution
- Alcoholic Iodine Solution
- Ambroxol Hydrochloride + Guaiphenesin + Terbutaline Sulphate Syrup
- Carbocisteine Syrup
- Lactulose Solution syrup
- Cefadroxil for Oral Suspension
- Amoxicillin for Oral Suspension
- Aciclovir Solution
- Ampicillin for Oral Suspension
- Barium Sulfate Suspension
- Azithromycin for Oral Suspension
- Ampicillin and Cloxacillin for Oral Suspension
- Azithromycin for Oral Suspension
- Azithromycin for Oral Suspension
- Acetaminophen Oral Solution
- Amoxicillin for Oral Suspension
- Amantadine Hydrochloride Syrup
- Azithromycin Sachet Powder
- Amoxicillin and Clavulanate Potassium for Oral Suspension
- Carbamazepine Oral Suspension
- Barium Sulfate Suspension
- Amlodipine Oral Solution
- Acyclovir Oral Suspension
- Albendazole Oral Suspension
- Metronidazole Oral Suspension
- Ambroxol Hydrochloride Syrup
- Amoxicillin for Oral Suspension
- Nystatin Oral Syrup
- Furazolidone Oral Suspension
- Paracetamol and Vitamin C Sachet
- Domperidone Oral Drops
- Co-trimoxazole oral Suspension
- Roxithromycin Oral Suspension
- Pyrantel Pamoate Oral Suspension
- Promethazine + paracetamol Oral Suspension
- Silymarin With Vitamin B-Complex Oral Suspension
- Simethicone Dill Oil And Fennel Oil Oral Drops
- Prednisolone Oral Solution
- Primidone Suspension
- Vitamin B Complex Syrup
- Sucralfate Suspension
- Salbutamol Syrup
- Sucralfate and Oxetacaine Suspension
- Potassium Chloride Oral Solution
- Simethicone + Dill Oil + Fennel Oil Oral Drops
- Simethicone oral Suspension
- Oflloxacin Suspension
- Sodium Valproate Oral Solution
- Terbutaline Sulphate + Bromhexince Hcl + Guaiphenesine + Menthol Syrup
- sucralfate oral suspension
- Salbutamol with Ambroxol Hcl Syrup
- Vitamin E Oral Drops
- Vitamin C Syrup
- Vitamin A Suspension
- Sodium Valproic Acid Solution
- Rifampicin Syrup
- Zinc Syrup
- Ascorbic Acid Syrup
- Promethazine Hydrochloride Syrup
- Vitamin B Complex Syrup
- Sodium Feredetate Oral Solution
- Salbutamol Sulphate + Bromhexine Hcl and Guaifenesin and Menthol Syrup
- Vitamin B Complex Syrup
- Vitamin C Oral Drops
- Paracetamol + Promethazine Hcl Suspension
- Potassium Citrate and Citric Acid Oral Solution
- Sucralfate and Oxetacaine Suspension
- Prednisolone Syrup
- Roxithromycin Oral Suspension
- Promethazine Syrup
- Sodium Picosulfate Syrup
- Valproic Acid Solution
- Oflloxacin & Tinidazole Suspension
- Posaconazole Oral Suspension
- Calcium Lactate, Calcium Gluconate And Vitamin D3 Syrup
- Metronidazole Oral Suspension
- Paracetamol Oral Suspension
- Multivitamin With Multimineral & Antioxidant Syrup
- Iodine and Iodide Solution
- Levamisole Hydrochloride Syrup
- Ketotifen Syrup
- Nevirapine Oral Suspension
- Lopinavir and Ritonavir Oral Solution
- Itraconazole oral solution
- Magaldrate & Simethicone, Simethicone Syrup
- Ipratropium Bromide Respirator Solution
- Levocetirizine Syrup
- Ipecacuanha Tincture
- Ivermectin Oral Solution
- Mebendazole Suspension
- Ipratropium Bromide Respirator Solution
- Paracetamol Oral Solution
- Paracetamol Paediatric Oral Solution
- Phenoxymethylpenicillin Dry syrup
- Kof Mute Syrup
- Iron Polymaltose Syrup
- Paracetamol Sachet
- Paracetamol Syrup
- Mebendazole Oral Suspension
- Multivitamin Multiminerals Syrups
- Potassium Chloride Oral Solution
- Paracetamol Oral Solution
- Veterinary Multivitamin syrup
- Naproxen Suspension
- Paracetamol and Ibuprofen Suspension
- Hydrogen Peroxide Solution
- Paracetamol Oral Solution
- Ketotifen Syrup
- Chlorpheniramine maleate +Paracetamol +Phenylephrine Suspension
- Norfloxacin Syrup
- Metronidazole Benzoate Suspension
- Omeprazole for oral suspension
- Paracetamol Suspension
- Lactulose Solution
- Nitrofurantoin Suspension
- Multivitamin Drops
- Paracetamol and Ibuprofen Suspension
- Magnesium Trisilicate Syrup
- Ibuprofen Oral Suspension
- Metoclopramide Oral Solution
- Phenobarbitone Syrup
- Multivitamin Syrup
- Lincomycin Syrup
- Amino Acid Vitamin And Mineral Syrup
- Piracetam Oral Solution
- Lactulose Solution
- Iron Syrup
- Multivitamin + Lysine Syrup
- Ibuprofen Oral suspension
- Phenoxymethylpenicillin Dry syrup
- Multivitamin Syrup
- Hyoscine Butylbromide Oral Suspension
- Paediatric Digoxin Oral Solution
- Ondansetron Solution
- Norfloxacin + Metronidazole Suspension
- Oxomemazine Syrup
- Lactulose Solution
- Omeprazole and Sodium Bicarbonate Suspension
- Paracetamol Drops
- Paidoterin Syrup
- Lopinavir and Ritonavir Oral Solution
- Ofloxacin + Ornidazole Suspension
- Multivitamin Syrup
- Amoxycillin & Potassium Clavulanate Syrup
- Anti cold Syrup
- Cough Syrup
- Ambroxol with Dextromethorphan Syrup
- Azithromycin Dry Syrup
- Ciprofloxacin Dispersible Syrup
- Sucralfate Suspension
- Albendazole Oral Suspension
- Cefixime For Oral Suspension
- Cefuroxim Axetil For Oral Suspension
- Amoxicillin Syrup
- Cefixime for Oral Suspension
- Amoxicillin Clavalunic acid suspension
- Amoxicillin Syrup
- Amoxicillin + Potassium Clavulanate Syrup
- Amoxicillin Clavalunic acid suspension
- Pharmaceutical Tablets
- Enalapril Tablets
- N-Acetyl Cysteine Tablets
- Bethanechol Tablets
- Pharmaceutical Tablets
- Volibris Tablets
- Methylergonovine Maleate Tablets
- Caffeine Extend Release Table
- Paracetamol And Caffeine Tablets
- Amlodipine Besylate and Atenolol Tablets
- Rosuvastatin Tablets
- Paracetamol Effervescent Tablets
- Cefixime Tablet
- Clarithromycin Tablets
- Aceclofenac + Paracetamol + Chlorzoxazone Tablets
- Candesartan Cilexetil Tablets
- Activated Charcoal Tablets
- Ambroxol Hydrochloride Tablets
- Amiloride and Hydrochlorothiazide Tablets
- Captopril and Hydrochlorothiazide Tablets
- Clonidine Tablets
- Gliclazide Tablets
- Naproxen Tablets
- Acetaminophen with Dicyclomine Tablets
- Nimodipine Tablets
- Oxcarbazepine Tablets
- Rabeprazole Tablets
- Potassium Iodate Tablet
- Quetiapine Sr Tablets
- Chlorthalidone Tablets
- Warfarin Sodium Tablets
- Warfarin Sodium Tablets
- Paracetamol Effervescent Tablets
- Paracetamol and Hyoscine Butylbromide Tablets
- Amiloride Tablets
- Metoprolol Hydrochlorthiazide Tablets
- Nicoumalone Tablets
- Praziquantel Tablets
- Nifedipine LA Tablets
- Paracetamol and Diclofenac Potassium Tablets
- Salbutamol Tablets
- Paracetamol and Hyoscine Butylbromide Tablets
- Ribavirin Tablets
- Mexiletine Capsules
- Nelfinavir Mesilate Tablets
- Citicoline Tablets
- Colchicine Tablets
- Allopurinol Tablets
- Citicoline and Piracetam Tablets
- Colchicine Tablets
- Clozapine Tablets
- Clozapine Tablets
- Lamivudine Tablets
- Fenofibrate Tablets
- Telmisartan and Hydrochlorothiazide Tablets
- Amlodipine Besylate and Lisinopril Tablets
- Betamethasone Tablets
- Lamivudine + Zidovudine + Nevirapine Tablets
- Cefpodoxime Proxetil Tablets
- Ramipril Tablets
- Nitazoxanide Tablet
- Iron Polymaltose Complex Tablets
- Terbutaline Sulfate Tablets
- Ibuprofen And Paracetamol Tablets
- Hydrochlorothiazide Tablets
- Etoricoxib Tablets
- Gabapentin Tablets
- Pyridoxine Hydrochloride Tablets
- Valsartan Tablets
- Theophylline Tablets
- Carbocisteine Capsules
- Cyclosporine Capsules
- Quetiapine Fumarate Tablets
- Quetiapine Tablets
- Quetiapine Tablets
- Sulpiride Tablets
- Donepezil Hydrochloride Tablets
- Propranolol Hydrochloride Tablets
- Propranolol Hydrochloride Tablets
- Thiamine Hydrochloride Tablets
- Sulpiride Tablets
- Pyridostigmine Bromide Tablets
- Thiamine Hydrochloride Tablets
- Sodium Valproate And Valproic Acid Cr Tablets
- Quetiapine Fumarate Tablets
- Pyridostigmine Bromide Tablets
- Donepezil Hydrochloride Tablets
- Pyridoxine Hydrochloride Tablets
- Propranolol Hydrochloride Tablets
- Warfarin Sodium Tablets
- Ramipril and Hydrochlorothiazide Tablets
- Ramipril and Hydrochlorthiazide Tablets
- Valsartan Tablets
- Sodium Valproate and Valproic Acid CR Tablets
- Quetiapine Tablets
- Pyridostigmine Bromide Tablets
- Procyclidine Hydrochloride Tablets
- Warfarin Sodium Tablets
- Propranolol Hydrochloride Tablets
- Thiamine Hydrochloride Tablets
- Ramipril and Hydrochlorothiazide Tablets
- Theophylline Tablets
- Thiamine Hydrochloride Tablets
- Valsartan Tablets
- Zinc Sulphate Dispersible Tablets
- Procyclidine Hydrochloride Tablets
- Methylcobalamin Tablets
- Pirfenidone Tablets
- Naproxen Sodium Tablets
- Oxcarbazepine Tablets
- Montelukast Sodium Tablets
- Nebivolol Tablets
- Phenazopyridine Hydrochloride Tablets
- Potassium Chloride Extended-Release Tablets
- Nifedipine and Atenolol Tablets
- Metoprolol Succinate Tablets
- Mepacrine Hydrochloride Tablet
- Nebivolol Tablets
- Methylcobalamin Tablets
- Noscapine Tablets
- Montelukast and Levocetirizine Hydrochloride Tablets
- Pantoprazole Sodium Tablets
- Methylergometrine Maleate Tablets
- Prazosin Tablets
- Naproxen Sodium Tablets
- Methylprednisolone Tablets
- Phenazopyridine Hydrochloride Tablets
- Pheniramine Maleate Tablets
- Papaverine Hydrochloride Tablets
- Nifedipine Extended-Release Tablets
- Phenindione Tablets
- Pheniramine Maleate Tablets
- Potassium Permanganate tablets
- Montelukast Sodium Tablets
- Papaverine Hydrochloride Tablets
- Oxytetracycline Tablets
- Methylcobalamin Tablets
- Naltrexone Hydrochloride Tablets
- Nifedipine Extended-Release Tablets
- Potassium Iodine Tablet
- Meloxicam Tablets
- Methylcobalamin + Alpha Lipoic Acid + Vitamin B1 + Vitamin B6 + Folic Acid Tablets
- Oxcarbazepine Tablets
- Piracetam Tablets
- Prazosin Tablets
- Montelukast and Levocetirizine Hydrochloride Tablets
- Montelukast Sodium Tablets
- Naproxen Sodium Tablets
- Piracetam Tablets
- Pirfenidone Tablets
- Metoprolol Tablets
- Piracetam Tablets
- Nimesulide Tablets
- Methylprednisolone Tablets
- Naproxen Tablets
- Minoxidil Tablets
- Metoprolol Succinate SR Tablets
- Meloxicam Tablets
- Meloxicam Tablets
- Minoxidil Tablets
- Pantoprazole Delay Released Tablets
- Piroxicam Dispesable Tablets
- Metoprolol Succinate SR Tablets
- Metoprolol Succinate SR Tablets 50
- Nifedipine Tablets
- Nifedipine Extended-Release Tablet
- Potassium Permanganate tablets
- Minoxidil Tablets
- Potassium Chloride Extended-Release Tablets
- Oral Rehydration Salts
- Aminophylline Tabelts
- Zinc Acetate Tablets
- Candesartan Cilexetil And Hydrochlorothiazide Tablets
- Phloroglucinol and Trimethylphloroglucinol Tablets
- Chlorthalidone Tablets
- Thiamine Hydrochloride Tablets
- Cimetidine Tablets
- Domperidone Tablets
- Diloxanide Furoate Tablets
- Ursodeoxycholic Acid Tablet
- Nitazoxanide Tablets
- Otilonium Bromide Tablets
- Magnesium Tablets
- Caffeine phenylephrine with Cetrizine Tablets
- Alfuzosin Tablets
- Chlorpheniramine Tablets
- Pre And Probiotic Capsule
- Secnidazole Tablets
- Loperamide Capsules
- Ticarcillin and Clavulanic acid injection
- Primidone Tablets
- Nicoumalone Tablets
- Oflloxacin & Tinidazole Tablets
- Thioridazine Hcl Tablets
- Thioridazine Hcl Tablets
- Thioridazine Hcl Tablets
- Nicoumalone Tablets
- Lopinavir & Ritonavir Tablets
- Nicoumalone Tablets
- Primidone Tablets
- Primidone Tablets
- Primidone Tablets
- Nicoumalone Tablets
- Primidone Tablets
- Primidone Tablets
- Primidone Tablets
- Lopinavir & Ritonavir Tablets
- Ofloxacin & Tinidazole Tablets
- Pyridoxine Hydrochloride Tablets
- Griseofulvin Tablets
- Acetylsalicylic Acid + Caffeine Tablets
- Rabeprazole Tablets
- Paracetamol , Ascorbic Acid Phenylephrine Hydrochloride And Pheniramine Maleate Effervescent Tablets
- Valacyclovir Tablets
- Amoxycillin Tablets
- Cetrizine Tablets
- Teneligliptin Tablets
- Tinidazole Tablets
- Ciprofloxacin Hydrochloride Tablets
- Combipack of Artesunate Tablets And Amodiaquine Hydrochloride Tablets
- Pioglitazone Hydrochloride Tablets
- Moxifloxacin Tablets
- Ciprofloxacin Tablets
- Diclofenac Sodium Tablets
- Levofloxacin Tablets
- Sparfloxacin Tablets
- Ibuprofen Tablets
- Ofloxacin and ornidazole Tablets
- Diclofenac Sodium Tablets
- Dihydroartemisinin And Piperaquine Phosphate Tablets
- Vitamin B12 Tablets
- Combikit of Sulfadoxine Pyrimethamine Tablets And Artesunate Tablet
- Favipiravir Tablets
- Methotrexate Tablets
- Rapamune Tablets
- Acotiamide Tablets
- Adempas Tablets Riociguat
- Dexamethasone Tablets
- Stivarga Tablets
- Giotrif Tablets Afatinib
- Bosentas Tablets
- Azithromycin Tablets
- Vitamin B1 Tablets
- Vitamin B6 Tablets
- Ibuprofen Paracetamol and Caffeine Tablets
- Diclofenac Potasium & Paracetamol Tablets
- Erythromycin Stearate Tablets
- Norfloxacin Tablets
- Paracetamol And Diclofenac Sodium Tablets
- Azithromycin Tablets
- Orphenadrine Plus Paracetamol Tablets
- Ciprofloxacin Hydrochloride Tablet
- Indometacin Capsules
- Levofloxacin Tablets
- Etoricoxib Tablets
- Etoricoxib Tablets
- Diclofenac Potasium Tablets
- Combipack of Artesunate Tablets And Amodiaquine Hydrochloride Tablets
- Ibuprofen Tablets
- Ofloxacin Tablets
- Pirfect Tablets
- Tracleer Tablets Bosentan
- Pirfenex Tablet
- Dasatinib Tablets
- Incivo Tablets
- Ziverdo Kit
- Inlyta Tablets
- Opsumit Tablets
- Viekirax Tablets
- Pirfenidone Tablets
- Daklinza Tablets
- Topiramate Tablets
- Theophylline with Salbutamol Tablets
- Theophylline with Salbutamol Tablets
- Topiramate Tablets
- Fexofenadine Tablets
- Colchicine Tablets
- Desloratadine Tablets
- Loratadine with Ambroxol Tablets
- Azithromycin Tablets
- Levocetirizine with Montelukast tablets
- Probenecid Tablets
- Sulfinpyrazone Tablets
- Lesinurad Tablets
- Salbutamol Tablets
- Fexofenadine Tablets
- Topiramate Tablets
- Calcium with Vitamin D3 Tablets
- Calcium with Vitamin D3 Tablets
- Carvedilol Tablets
- Candesartan Cilexetil and Hydrochlorothiazide Tablets
- Candesartan Cilexetil Tablets
- Carvedilol Tablets
- Carvedilol Tablets
- Carisoprodil Tablets
- Calcium Gluconate Tablets
- Aripiprazole Tablets
- Calcium Citrate Tablets
- Ascorbic Acid Tablets
- Carbamazepine Extended Release Tablets
- Captopril Tablets
- Montelukast and Levocetirizine Hydrochloride Tablets
- Nifedipine Tablet
- Methocarbamol Tablets
- Methocarbamol Tablets
- Metoprolol Tablets
- Hydralazine Tablets
- Ivermectin Tablets
- Labetalol Hydrochloride Tablets
- Hydralazine Tablets
- Gastro Resistant Sodium Valproate Tablets
- Ibuprofen Tablets
- Drotaverine hydrochloride Tablets
- Griseofulvin Tablets
- Felodipine Tablets
- Felodipine Tablets
- Folic Acid Tablets
- Isoniazid Tablets
- Folic Acid Tablets
- Glipizide Tablets
- Fenbendazole Tablets
- L- Glutamine Tablets
- Haloperidol Tablets
- Glucosamine and Chondroitin Tablets
- Labetalol Hydrochloride Tablets
- Etamsylate Tablets
- Ivermectin Tablets
- Mefenamic Acid with Paracetamol Tablets
- Disulfiram Tablets
- Drotaverine hydrochloride tablets
- Etamsylate Tablets
- Ketorolac Tablets
- Lamotrigine Tablets
- Enalapril Maleate Tablets
- Everolimus Tablets
- Enalapril Tablets
- Mefenamic Acid with Paracetamol Tablets
- Folic Acid Tablets
- Eperisone Hydrochloride Tablets
- Ibuprofen Tablets
- Famotidine Tablet
- Mefenamic Acid Tablets
- Haloperidol Tablets
- Enalapril Maleate Tablets
- Haloperidol Tablet
- Hydralazine Tablets
- Gastro Resistant Sodium Valproate Tablets
- Drotaverine hydrochloride tablets
- ketotifen Tablets
- Drotaverine and Paracetamol Tablets
- Furosemide Tablets
- Etoricoxib Tablets
- Famotidine Tablet
- Labetalol Hydrochloride Tablets
- Enalapril Maleate Tablets
- Drotaverine and Paracetamol Tablets
- Glucosamine and Chondroitin Tablets
- Haloperidol Tablets
- Furosemide Tablets
- Ivermectin Tablets
- Folic Acid Tablets
- Efavirenz Tablets
- Duloxetine Hydrochloride Tablets
- Lamotrigine Tablets
- Disulfiram Tablets
- Lamotrigine Tablets
- Ivermectin Tablets
- Efavirenz Tablets
- Isoniazid Tablets
- Ibuprofen Tablets
- Etoricoxib Tablets
- Clomipramine Tablets
- Chlorpheniramine Maleate Tablets
- Chlorpromazine Hydrochloride Tablets
- Cinacalcet Hydrochloride Tablets
- Diclofenac Potassium Tablets
- Cinitapride Hydrogen Tablets
- Chlorthalidone Tablets
- Cimetidine Tablets
- Chlorpromazine Hydrochloride Tablets
- Chlorpromazine Hydrochloride Tablets
- Cinnarizine Tablet
- Clonidine Tablets
- Citicoline Tablets
- Cetirizine Hydrochloride Tablets
- Diclofenac Potassium Tablets
- Cimetidine Tablets
- Clonidine Tablets
- Cinacalcet Hydrochloride Tablets
- Ciprofloxacin HCl. Tablets
- Cimetidine Tablets
- Clomipramine Tablets
- Chlorthalidone Tablets
- Cinacalcet Hydrochloride Tablets
- Clomipramine Tablets
- Clonidine Tablets
- Citiolone and L-Cysteine Tablet
- Cinnarizine Tablet
- Cinnarizine Tablet
- Clozapine Tablets
- Chlorpheniramine Maleate Tablets
- Candesartan Cilexetil Tablets
- Arginine Tablets
- Calcium Lactate Tablets
- Calcium Carbonate Tablets
- Carbamazepine Tablets
- Bromhexine Hydrochloride Tablets
- Asenapine Tablets
- Ascorbic Acid Tablets
- Carbamazepine Tablets
- Ascorbic Acid Tablets
- Buspirone Hydrochloride Tablets
- Calcium with Vitamin D3 Tablets
- Captopril Tablets
- Candesartan Cilexetil and Hydrochlorothiazide Tablets
- Calcium Citrate and Vitamin D3 Tablets
- Calcium Lactate Tablets
- Calcium Folinate Tablets
- Ascorbic Acid Tablets
- Calcium and Colecalciferol Tablets
- Calcium Carbonate and Vitamin D3 Tablets
- Calcium with Vitamin D3 Tablets
- Arterolane Maleate and Piperaquine Phosphate Tablets
- Captopril Tablets
- Acetaminophen Tablets
- Aripiprazole Tablets
- Ascorbic Acid Tablets
- Carvedilol Tablets
- Captopril Tablets
- Calcium Citrate, Magnisium, Zinc , Vitamin D3 Tablets
- Ascorbic Acid Tablets
- Amiodarone Tablets
- Aceclofenac + Paracetamol Tablets
- Metronidazole Tablets
- Acetaminophen Tabelts
- Amisulpride Tablets
- Aceclofenac And Paracetamol Tablets
- Allopurinol Tablets
- Amlodipine and Losartan Potassium Tablet
- Albendazole Tablets
- Amlodipine and Atenolol Tablet
- Amlodipine and Lisinopril Tablets
- Alfacalcidol and Calcium Carbonate Tablets
- Amiloride Hcl Tablets
- Aciclovir Tablets
- Amlodipine + Atenolol Tablet
- Amlodipine + Perindopril Tablets
- Amlodipine besilate Tablet
- Amiloride Tablets
- Aceclofenac and Paracetamol and Serratiopeptidase Tablets
- Acetylsalicylic Acid Tablets
- Acetaminophen+Pseudoefedrine Hcl + Chlorpheniramine Maleate Tablets
- Oral Rehydration Salts
- Albendazole Chewable Tablets
- Aceclofenac + Paracetamol Tablets
- Amlodipine and Valsartan Tablets
- Amino Salicylic Acid Tablets
- Albendazole Chewable Tablets
- Acetylsalicylic Acid Tablets
- Amlodipine and Lisinopril Tablets
- Amlodipine and Valsartan Tablets
- Aceclofenac Tablets
- Acetylsalicylic Acid Tablets
- Acetaminophen with Dicyclomine Tablets
- Ambroxol Hydrochloride + Cetrizine Hydrochloride Tablet
- Amisulpride Tablets
- Albendazole Tablets
- Amlodipine and Losartan Potassium Tablet
- Amlodipine Besilate Tablet
- Amlodipine + Enalapril Maleate Tablets
- Alfacalcidol with Calcium Tablets
- Amlodipine Besilate Tablet
- Amiodarone Tablets
- Amlodipine + Atenolol Tablets
- Acetaminophen Tablets
- Amlodipine + Atenolol Tablets
- Allopurinol Tablets (Uncoated)
- Aminophylline Tabelts
- Albendazole+ Selenium + Cobalt
- Lopinavir & Rotonavir Tablets
- Acetaminophen With Dicyclomine Tablets
- Aciclovir Tablets
- Allopurinol Tablets
- Amlodipine and Valsartan Tablets
- Albendazole Chewable Tablets
- Amlodipine And Olmesartan Tablets
- Acetyl Salicylic Acid Dispersible Tablets
- Aminocaproic Acid Tablets
- Amlodipine and Lisinopril Tablets
- Amlodipine + Perindopril Tablets
- Amlodipine Besilate Tablet
- Paracetamol &Caffeine Effervescent Tablets
- Calcium Carbonate Effervescent Tablets
- Paracetamol Effervescent Tablets
- Vitamin C Effervescent Tablets
- Paracetamol & Vitamin C Effervescent Tablets
- Cefadroxil Tablets
- N-Acetyl Cysteine Effervescent Tablets
- Electrolyte Enhanced Drink Effervescent Tablets
- Diphenhydramine Hydrochloride Paracetamol Effervescent Tablets
- Paracetamol & Caffeine Effervescent Tablets
- Sodium Acid Phosphate Effervescent Tabets
- Cifixime and Ofloxacin tablets
- Paracetamol & Caffeine Effervescent Tablets
- Cephalexin Dispersible Tablets
- Paracetamol effervescebt Tablets
- Potassium Citrate Effervescent Tablets
- ORS Effervescent Tablet
- Cefadroxil Dispersible Tablets
- Rosuvastatin Tablets
- Cefuroxime Axetil Dispersible Tablets
- Lamivudine + Zidovudine Tablets
- Captopril Tablets
- Secnidazole Tablets
- Fenofibrate Tablets
- Oral Rehydration Salts
- Betahistine Hydrochloride Tablets
- Enalapril Tablets
- Cefixime And Clavulanate Potassium Tablets
- Rosuvastatin and Fenofibrate Tablets
- Sevelamer Hydrochloride Tablets
- Atorvastatin and Ezetimibe Tablets
- Telmisartan Tablets
- Betahistine Hydrochloride Tablets
- Lisinopril Tablets
- Irbesartan Tablets
- Amlodipine Besylate Tablets
- Cefixime Dispersible Tablets
- Salbutamol Sulphate And Theophylline Tablets
- Atorvastatin and Fenofibrate Tablets
- Amlodipine Besylate Tablets
- Cefpodoxime Proxetil and Clavulanate Tablets
- Cifixime and Clavulanate Potassium Dispersible Tablets
- Cefuroxime Axetil and Clavulanate potassium Tablets
- Gabapentin Tablets
- Diloxonide furoate Tablet
- Albendazole Tablets
- Tinidazole Tablets
- Telmisartan Tablets
- Dronedarone (as Hydrochloride) Tablets
- Dexamethasone Tablets
- Prednisolone Tablets
- Cefuroxime Axetil and Clavulanate potassium Tablets
- Atorvastatin Tablets
- Cifixime and Clavulanate Potassium Dispersible Tablets
- Tamsulosin hydrochloride (modified release) & Finasteride tablets
- Lamivudine + Zidovudine + Efavirenz Tablets
- Fenofibrate Tablets
- Clopidogrel Tablets
- Betahistine Hydrochloride Tablets
- Ramipril Tablets
- Rosuvastatin Tablets
- Irbesartan Tablets
- Oral Rehydration Salts and Zinc Sulphate Tablets
- Prednisolone Tablets
- Irbesartan + Hydrochlorthiazide Tablets
- Citicoline Tablets
- Ketoanalogue Plus Essential Amino Acids Tablet
- Sevelamer Carbonate Tablets
- Sevelamer Carbonate Tablets
- Cefpodoxime Proxetil Dispersible Tablets
- Lisinopril Tablets
- Methyldopa Tablets
- Deferasirox Tablets
- Tenofovir + Emticitabine Tablets
- Metronidazole Tablets
- Valsartan Tablets
- Levetiracetam Tablets
- Nevirapine Tablets
- Cefuroxime Axetil Tablets
- Cefixime Dispersible Tablet
- Cefpodoxime Proxetil Dispersible Tablets
- Sevelamer Hydrochloride Tablets
- Mebendazole Tablets
- Cefuroxime Axetil Tablets
- Cyproheptadin Tablets
- Tablets
- Bisacodyl Tablet
- Rupatadine Tablets
- Prasugrel Tablets
- Citicoline Tablets
- Diclofenac Potassium Tablets
- Pharmaceutical Capsule
- Tamsulosin Hydrochloride Modified Release Capsules
- Gabapentin Capsules
- Cephalexin Capsules
- Duloxetine Capsules
- Diacerein Capsule
- Rifabutin Capsules
- Ibuprofen and Paracetamol and Caffeine Capsules
- Rifampin and Isoniazid Capsules
- Sulfur Ointment
- Vitamin E Capsule
- Vitamin A Capsules
- Pregabalin Capsule
- Flucloxacillin Sodium Capsules
- Succimer Capsule 100
- Pregabalin and Etoricoxib Capsule
- Mexiletine Hydrochloride Capsules
- Nifedipine Capsules
- Valsartan Capsule
- Piroxicam Capsules
- Nortriptyline Hydrochloride Capsule
- Thalidomide Capsule 200 mg
- Tamsulosin Capsule
- Pregabalin Capsule
- Pregabalin and Methylcobalamin Capsules
- Piracitam Capsules
- Mexiletine Hydrochloride Capsules
- Rabeprazole Sodium and Domperidone Capsule
- Rifampicin Capsules
- Nortriptyline Hydrochloride Capsule
- Ribavirin Capsules
- Valsartan Capsule
- Pregabalin Capsule
- Saquinavir Capsule
- Rifampicin Capsules
- Tetracycline Capsules
- Omeprazole and Sodium Bicarbonate Capsules
- Tenoxicam Capsules
- Stavudine Capsule
- Penicillamine capsules
- Valproic Acid Capsules
- Phenoxybenzamine Capsules
- Ramipril Capsules
- Omeprazole Capsules
- Ribavirin Capsules
- Saw Palmetto Capsule
- Ramipril Capsules
- Flucloxacillin Sodium Capsules
- Flucloxacillin Capsules
- Stavudine Capsule
- Norfloxacin Capsule
- Paracetamol Chlorzoxazone Capsule
- Nitrofurantoin Capsules
- Ribavirin Capsules
- Omeprazole capsules
- Paromomycin Sulfate Capsule
- Ramipril Capsules
- Mexiletine capsules
- Omeprazole Domperidone Capsules
- Pantoprazole Capsule
- Thalidomide Capsule 100 mg
- Ramipril Capsules
- Thalidomide Capsule 50 mg
- Flucloxacillin Capsules
- Tetracycline Capsules 250 mg
- Vitamin D Capsule
- Zidovudine Capsule
- Vitamin B Complex Capsules
- Xtandi Capsule Enzalutamide
- Paracetamol capsule
- Pantoprazole Sodium and Domperidone Capsules
- Albendazole Oral Suspension
- Cyproheptadine Lysine and Peptone Syrup
- Vitamin A Capsules
- Gastro-resistant Peppermint Oil Capsules
- Mefenamic Acid Capsule
- Indinavir Capsules
- Indinavir Capsules
- Indomethacin Capsules
- Lincomycin Capsules
- Ketoprofen Sr Capsule
- Pregabalin Capsule
- Glucosamine Capsules
- Methylcobalamin + Alpha Lapoic Acid + Pyridoxine Hydrochloride + Folic Acid Capsule
- Vitamin E Capsule
- Mexiletine capsules
- Norfloxacin Capsule
- Omeprazole capsules
- Loperamide Hydrochloride Capsules
- Iodised Oil Capsule
- Hydroxy Methylbutyrate Capsule
- Methylcobalamin + Alpha Lapoic Acid + Pyridoxine Hydrochloride + Folic Acid Capsule
- Itraconazole Capsule
- Miglustat Capsules
- Vancomycin Hydrochloride Capsules
- Lansoprazole and Domperidone Capsule
- Gabapentin Capsules
- Vitamin A Capsules
- Etodolac Capsules
- Ritonavir Capsule
- Flucytosine Capsule
- Vitamin A Capsule
- Ketoprofen SR Capsule
- Omeprazole + Domperidone Capsules
- Mefenamic Acid Capsule
- Indomethacin Capsules
- Etodolac Capsules
- Clindamycin Capsules
- Calcitriol Capsules
- Doxycycline Hydrochloride Capsules
- Dantrolene Sodium Capsule
- Carbamazepine Capsule
- Esomeprazole Delayed Release Capsule
- Diclofenac Colestyramine Capsule
- Azithromycin Capsules
- Cyclosporine Capsules
- Clofazimine Capsules
- Deferiprone Capsule
- Cefaclor Capsules
- Cod Liver Oil Capsules
- Clindamycin Capsules
- Calcitriol Capsules
- Cefixime Capsule
- Efavirenz Capsule
- Dabigatran Etexilate Capsule
- Carbamazepine Capsule
- Cysteamine Bitartrate Capsule
- Co-fluampicil Capsules
- Cefaclor Capsules
- Azithromycin Capsules
- Efavirenz Capsule
- Carbocisteine Capsules
- Efavirenz Capsule
- Dicloxacillin Sodium Capsules
- Cefixime Capsule
- Emtricitabine Capsule
- Clofazimine Capsules
- Esomeprazole Delayed Release Capsule
- Cysteamine Bitartrate Capsule
- Dabigatran Etexilate Capsule 110
- Nifedipine Capsules
- Cyclosporine Capsules
- Vitamin B Complex Capsules
- Vitamin B Complex Capsules
- Rifampicin Capsules
- Succimer Capsule
- Pharmaceutical Capsules
- Racecadotril Capsules
- Ketoprofen Capsules
- PANTOPRAZOLE WITH DOMPERIDONE CAPSULE
- Victrelis Capsules
- Imnovid Capsules
- Celecoxib Capsules
- Lincomycin Capsule
- Celecoxib Capsules
- Doxycycline Capsules
- Olysio Capsule
- Zavesca Capsule
- Tisinon Capsule
- Cefaclor Capsules
- Calcitriol Capsules
- Ketoprofen Capsules
- Furazolidone Capsules
- Duloxetine Capsules
- Ganciclovir Capsules
- Ganciclovir Capsules
- Ketoprofen Capsules
- Calcium Dobesilate Capsules
- Niflumic Acid Capsule
- Rabeprazole Sodium & Domperidone Capsule
- Alfacalcidol Capsule
- Alfacalcidol Capsule
- Amantadine Capsule
- Cephalexin Capsules
- Ampicillin + Cloxacillin Capsules
- Amoxicillin Capsules
- Cefdinir Capsules
- Amoxicillin Capsules
- Piroxicam Capsules
- Suppositories
- Multivitamin Tablets
- Calcium Carbonate With Vitamin D3 Tablets
- Multi vitamin Capsules
- Multivitamin with Antioxident and minerals 1
- Multivitamin with Antioxident and minerals 3
- Multivitamin and Mineral Tablets - 2
- Multivitamin with Antioxident and minerals 2
- Multivitamin and Mineral Tablets - 4
- Multivitamin and Mineral Tablets - 3
- Multivitamin and Minerals Tablets -1
- Antibiotic Infusions
- Caffeine Tablet
- Multivitamin & Minerals Effervescent Tablets
- Multivitamin tablets
- Multivitamin capsules
- Ferrous Sulphate And Folic Acid
- Folic Acid Tablets
- Calcium Carbonate With Vitamin Tablets
- Hormonal & Steroidal Products
- Megestrol Acetate Tablets
- Dehydropiandrosterone Tablets
- Megestrol Acetate Tablets
- Megestrol Acetate Tablets
- Methylprednisolone Tablets
- Enrofloxacin injection
- Carboprost Trometamol Injection
- Progesterone Capsule
- Clomid Tablets
- Vasopressin Injection
- Vasopressin Injection
- Vasopressin Injection
- Stanozolol Injection
- Stanozolol Injection
- Hucog 5000 Injection
- Danazol Capsules
- Danazol Capsules
- Danazol Capsules
- Cabergoline Tablets
- Medroxyprogesterone Tablets
- Hcg Injection
- Methylprednisolone Injection
- Dexamethasone Tablets
- Prednisolone Tablet
- Norethisterone Tablets
- Desogestrel Tablets
- Clomifene Tablets
- Clomiphene Citrate Tablets
- Oxymethalone Tablets
- Levonorgestrel Tablets
- Dehydroepiandrosterone with Folic Acid Capsules
- Nandrolone Decanoate Injection
- Stanozolol Tablets
- Stanozolol Injections
- Nandrolone cypionate Injection
- Drostanolone Propionate Injection
- Methandienone Tablets
- Highly Purified Chorionic Gonadotropin Injections
- Antibiotic Tablets
- Amoxicillin+ Clavalunic acid Tablets
- Cefixime Dispersible Tablets
- Cefalexin Capsules
- Cepodoxine Tablets
- Tenofovir Alafenamide Tablets
- Levofloxacin Tablets
- Trimethoprim And Sulphamethoxazole Tablets
- Cefixime Tablets
- Zidovudine Tablets
- Trimethoprim and Suphamethoxazole Tablets
- Zidovudine Tablets
- Erythromycin Estolate Tablets
- Erythromycin Estolate Tablets
- Norfloxacin Dispersible Tablets
- Norfloxacin Tablets
- Oxytetracycline Tablets
- Nalidixic Acid Tablets
- Amoxicillin Tablets
- Faropenem Tablets
- Cefixime Tablets
- Ornidazole Tablets
- Antibiotic Medicines
- Erythromycin Tablets
- Ofloxacin Ornidazole Tablets
- Clarithromycin Tablets
- Roxithromycin Tablets
- Cloxacillin Capsules
- Metronidazole Tablets
- Ciprofloxacin Tablets
- Cefpodoxime Proxetil With Pottasium Clavulanate Tablets
- Levofloxacin Tablet
- Doxycycline Capsules
- Amoxycillin Tablets
- Azithromycin Tablets 500mg
- Amoxicillin + Clavalunic Acid Tablets
- Acriflavine Powder
- Cefpodoxime Tablets
- Cefaclor Tablets
- Rifaximin Tablets
- Clindamycin Capsules
- Doxycycline Hyclate Capsules
- Ciprofloxacin Tablets
- Lincomycin Capsule
- Amoxicillin and Potassium Clavulanate Tablets
- Amoxicillin and Potassium Clavulanate Tablets
- Artesunate + Amodiaquine tablets
- Ampicillin and Cloxacillin Capsules
- Cefadroxil Tablets
- Amoxicillin And Potassium Clavulanate Tablets
- Amoxicillin Dispersible Tablets
- Cefadroxil Tablets
- Ampicillin Capsules
- Azithromycin Tablet
- Cefadroxil Dispersible Tablets
- Cefalexin Capsules
- Erythromycin Tablets
- Entecavir Tablets
- Erythromycin Tablets
- Enrofloxacin Tables
- Enrofloxacin Tables
- Ciprofloxacin Tablets
- Ciprofloxacin Tablets
- Metronidazole Tablets
- Ciprofloxacin Tablets
- Cefpodoxime and Ofloxacin Tablets
- Ciprofloxacin+Tinidazole Tablets
- Chlorambucil Tablets
- Cefixime Tablets
- Cephalexin Tablets
- Metronidazole Tablets
- Cefuroxime Axetil and Clavulanic acid Tablets
- Metronidazole Tablets
- Ciprofloxacin HCl. Tablets
- Dicloxacillin Capsules
- ciprofloxacin Tablets
- Ciprofloxacin+Tinidazole Tablets
- Cefpodoxime Proxetil Tablets
- Chloroquine Phosphate Tablet
- Cephalexin Tablets
- Cefpodoxime and Ofloxacin Tablets
- Ciprofloxacin+Tinidazole Tablets
- Cefprozil Tablets
- Cefixime Tablets
- Ciprofloxacin Tablets
- Cefpodoxime Proxetil Dispersible Tablets
- Cloxacillin Capsules
- Cefixime Tablets
- Cefuroxime Axetil and Clavulanic acid Tablets
- Cefpodoxime Proxetil Tablets
- Cefixime And Ofloxacin Tablets
- Cloxacillin Capsules
- Cefpodoxime and Clavulanic Acid Tablets
- Cefixime Tablets
- Cefuroxime Axetil Tablets
- Cefpodoxime Proxetil Dispersible Tablets
- Clarithromycin Modified Release Tablets
- Cefuroxime Axetil Tablets
- Cefpodoxime Proxetil Tablets
- Cefuroxime Axetil and Clavulanic acid Tablets
- Cefuroxime Axetil and Clavulanic acid Tablets
- Cefuroxime Axetil Dispersible Tablets
- Cephalexin Capsules
- Ciprofloxacin+Tinidazole Tablets
- Chlorambucil Tablets
- Cefpodoxime + Clavulanic Acid Tablets
- Cefixime with Clavulanate Potassium Tablet
- Cefixime with Lactobacillus Tablets
- Amoxicillin Tablets
- Amoxicillin Capsules
- Cefalexin Capsules
- Ampicillin Trihydrate Tablets
- Cefaclor Tablets
- Azithromycin Tablet
- Cefaclor Sustain Release Tablets
- Amoxicillin Capsules
- Amoxicillin Tablets
- Amoxicillin Tablets
- Azithromycin Tablets
- Cefadroxil Tablets
- Ampicillin and Cloxacillin Capsules
- Amoxicillin Capsules
- Amoxicillin Trihydrate and Clavulanic Acid Tablets
- Cefaclor Tablets
- Ampicillin Capsules
- Azithromycin Tablets
- Ofloxacin + Ornidazole Tablets
- Neomycin Sulfate Tablets
- Amoxicillin and Cloxacillin with Lactobacillus Capsules
- Amoxicillin and Clavulanate potassium Tablets
- Amoxicillin and Cloxacillin Capsules
- Cefadroxil Clavulanic Acid Tablets
- Amoxicillin And Clavulanate Potassium Disp. Tablets
- Amoxicillin + Clavulanate potassium Tablets
- Amoxicillin and Clavulanate Potassium Disp. Tablets
- Amoxicillin + Potassium Clavulanate Tablets
- Amoxicillin Trihydrate Dispersible Tablet
- Amoxicillin Trihydrate Capsules
- Amoxicillin dispersible Tablets
- Ampicillin Dispersible Tablets
- Ofloxacin Tablet
- Norfloxacin Tablets
- Immunization & Vaccination Drugs
- TD Vaccine
- Influenza Vaccines
- HIB Vaccine
- Cholera Vaccines
- Human Rabies Immunoglobulin Vaccines
- Typhoid Vaccine
- Bcg Vaccine
- Meningitis Vaccine
- AbhayRIG Rabies Antiserum
- Rotavirus vaccine
- Snake Venom Antiserum
- DPT Vaccine
- Rabipur Vaccines
- Vaxtar 5
- Imovax Vaccine
- Hepatitis A Vaccine
- Hydrocortisone Sodium Injection
- Avaxim Vaccine
- Tetanus Toxoid Vaccines
- Anti Rabies Vaccine
- Energix B Vaccine
- Hepatitis B vaccine
- Abhayrab Vaccine
- Rabies Antiserum Immunoglobulin
- Oral Polio Vaccine
- MMR Vaccine
- Antibiotic Injections
- Cefoperazone, Sulbactam Injection
- Cefotaxime Sodium Injection
- Ceftazidime Injection
- Colistimethate Sodium For Injection
- Ciprofloxacin Injection
- Meropenem for Injection
- Ceftriaxone and Tazobactam for Injection
- Cefoxitin Sodium Injection
- Tobramycin Injection
- Penicillin G Injection
- Ampicillin Injection
- Flucloxacillin Sodium Injection
- Sterile Water For Injections
- Meropenem injection
- Cefoxitin Injection
- Gentamicin Sulphate Injection
- Gentamicin Sulphate Injection
- Gentamicin Injection
- Gentamicin Injection
- Fortified Procaine Penicillin For Injection
- Flucloxacillin for Injection
- Gentamicin Injection
- Gentamicin Injection
- Gentamicin Sulphate Injection
- Gentamicin Injection
- Haloperidol Decanoate Injection
- Erythromycin Lactobionate for Injection
- Gentamicin Sulphate Injection
- Fortified Procaine Penicillin For Injection
- Doxycycline For Injection
- Doxycycline For Injection
- Gentamicin Injection
- Diphtheria Antitoxin Injection
- Colistimethate Sodium For Injection
- Ceftiofur Sodium Sterile Powder For Injection
- Clarithromycin For Injection
- Ceftiofur Sodium Sterile Powder For Injection
- Ceftazidime Injection
- Cloxacillin Injection
- Ceftriaxone for Injection
- Clindamycin Injection
- Ceftriaxone for Injection
- Ceftizoxime Injection
- Ceftazidime Injection
- Clindamycin and Dextrose Injection
- Cloxacillin Sodium for Injection
- Cefradine For Injection
- Colistimethate Sodium For Injection
- Ceftriaxone and Tazobactam for Injection
- Colistimethate Sodium For Injection
- Cloxacillin Injection
- Cefuroxime for Injection
- Cefpirome Sulfate Injection
- Ceftizoxime Injection
- Cefuroxime Sodium for Injection
- Cefuroxime for Injection
- Ceftriaxone for Injection
- Cloxacillin Injection
- Ceftriaxone and Tazobactam for Injection
- Ceftriaxone with Sulbactam For injection
- Clindamycin Injection
- Ceftriaxone with Sulbactam for injection
- Cefradine For Injection
- Ceftriaxone and Tazobactam for Injection
- Ceftriaxone for Injection
- Ceftizoxime Injection
- Cefuroxime for Injection
- Ceftriaxone Sodium Sterile for Injectio
- Ceftizoxime Injection
- Clindamycin and Dextrose Injection
- Ceftazidime Injection
- Ciprofloxacin Injection
- Ceftizoxime Sodium For Injection
- Clindamycin Injection
- Ceftriaxone and Tazobactam for Injection
- Aztreonam For Injection
- Benzathine Penicillin Injection
- Cefepime for Injection
- Cefepime for Injection
- Benzathine Penicillin Injection
- Cefotaxime Sodium Injection
- Ampicillin Sodium For Injection
- Aztreonam For Injection
- Bupivacaine Hcl Injection
- Ampicillin Sodium For Injection
- Cefepime for Injection
- Calcium Gluconate Injection
- Aztreonam For Injection
- Aztreonam For Injection
- Cefoxitin Injection
- Cefoperazone + Sulbactam For Injection
- Cefoperazone and Sulbactam Injection
- Azithromycin For Injection
- Cefepime and Tazobactam Injection
- Benzathine Benzyl Penicillin Injection
- Ampicillin Sodium For Injection
- Cefepime Hcl Injection
- Cefotaxime Sodium For Injection
- Cefotaxime Sodium Injection
- Benzathine Penicillin Injection
- Cefepime and Tazobactam injection
- Anti D-Immunoglobulin Injection
- Aztreonam For Injection
- Benzathine Benzyl Penicillin Injection
- Flucloxacillin Sodium Injection
- Clindamycin and Dextrose Injection
- Oxacillin for Injections
- Cloxacillin Injections
- Cefepime Injection
- Benzyl Penicillin Injection
- Ceftriaxone Sulbactam Injection
- Cephalothin Injection
- Cefpirome Injection
- Nafcillin Injection
- Cefpirome Injection
- Gentamycin Injection
- Cefoperazone Injection
- Antibiotic Injections
- Ampicillin Salbactum Injection
- Ceftriaxone with Salbactam for injection
- Ampicillin and Sulbactam For Injection
- Ceftriaxone with Salbactam for injection
- Ceftriaxone for Injection
- Ceftriaxone and Sulbactam for Injection
- Ceftriaxone Sodium + Sulbactam Sodium For Injection
- Tobramycin Sulfate Injection
- Ceftriaxone Sodium For Injection
- Amikacin Injection
- Oxytetracycline Injection
- Ampicillin and Cloxacillin For Injection
- Ampicillin and Cloxacillin For Injection
- Cyanocobalamin Injection
- Ceftriaxone with Salbactam For injection
- Ampicillin and Cloxacillin For Injection
- Ampicillin and Sulbactam For Injection
- Ceftriaxone + Sulbactam For Injection
- Cefoperazone Injection
- Ampicillin + Sulbactam For Injection
- Ampicillin and Cloxacillin For Injection
- Sterile Co-Trimoxazole Injection
- Ampicillin and Cloxacillin For Injection
- Amoxicillin Potassium Clavulanic Acid Injection
- Ampicillin and Cloxacillin For Injection
- Ampicillin + Sulbactam For Injection
- Oxytetracycline Injection
- Amoxycillin and Dicloxacillin Injection
- Amoxicillin and Potassium Clavulanate For Injection
- Amphotericin B for Injection
- Tobramycin Injection
- Ampicillin and Cloxacillin For Injection
- Enrofloxacin injection
- Cyanocobalamin Injection
- Amoxicillin With Sulbactum For Injection
- Amoxicillin With Cloxacillin For Injection
- Tobramycin Sulfate Injection
- Oxytetracycline Hydrochloride Injection
- Ceftriaxone for Injection
- Ampicillin and Sulbactam For Injection
- Cefoperazone Sodium and Sulbactam Sodium Injection
- Cefoperazone Sodium Injection
- Amikacin Sulphate Injection
- Amoxicillin Sodium For Injection
- Cefoperazone Sodium and Sulbactam Sodium Injection
- Amikacin Sulphate Injection
- Amikacin Sulphate Injection
- Tigecycline For Injection
- Cefepime Injection
- Amikacin Injection
- Antibiotic Injection
- Cefazolin Injection
- AMPICILLIN SALBACTUM INJECTION
- Ceftriaxone Sodium Injection
- Amikacin Injection
- Tigecycline Injection
- Tigecycline Injections
- Teicoplanin Injections
- Piperacillin Tazobactum Injections
- Amoxycillin and Potassium Clavulanate Injection
- Benzathine Penicillin Injections
- Ampicillin Sodium With Sulbactam Sodium For Injection
- Ampicillin Sodium With Sulbactam Sodium For Injection
- Tylosin Tartrate Injection
- Sodium Bicarbonate Injection
- Tylosin Tartrate Injection
- Teicoplanin For Injection
- Streptomycin for Injection
- Streptomycin for Injection
- Meropenem for Injection
- Lincomycin Injection
- Meropenem for Injection
- Imipenem and Cilastatin for Injection
- Imipenem and Cilastatin for Injection
- Meropenem for Injection
- Meropenem and Sulbactam Injection
- Imipenem and Cilastatin for Injection
- Gentamicin Injection
- Ampicillin Injection
- Ceftriaxone Sodium Injection
- Doxycycline Injection
- Cefoperazone Injection
- Amoxicillin and Potassium Clavulanate Tablets
- Benzathine Penicillin Injection
- Lincomycin Injection
- Anti D-Immunoglobulin Injection
- Inhaler, Rotacaps & Respules
- Aerocort Rotacaps
- Salbutamol Inhalation IP
- Budesal Respules
- Tiova Rotacaps
- Tobramycin Inhalation Solution
- Foracort Respules
- Salbutamol Inhaler
- Salbutamol Pressurised Inhalation
- Seroflo Inhaler
- Flohale Inhaler
- Tiotropium Bromide Inhaler
- Levolin Rotacaps
- Budecort Respules
- Levolin Inhaler
- Ipravent Inhaler
- Ipravent Rotacaps
- Ipravent Respules
- Maxiflo Inhaler
- Foracort Inhaler
- Levolin Respules
- Salbutamol Sulphate Rotacaps
- Seroflo Rotacaps
- Aerocort Inhaler
- Foracort Rotacaps
- Duolin Respules
- Paediatric syrup & Drops
- Cefixime Dry Syrup
- Cefaclor Oral Suspension
- Cefixime Dry Syrup
- Alfacalcidol Oral Drops
- Multivitamin minerals and lysine syrup
- Pharmaton Kiddi Syrup
- Infant Multivitamins Oral Drops
- Diclofenac Free Acid Suspension
- Cefpodoxime Proxetil Suspension
- Nimesulide Paracetamol Suspension
- Clarithromycin Oral Suspension
- Cefixime Oral Suspension
- Ibuprofen Oral Suspension
- Azithromycin Oral Suspension
- Erythromycin Estolate Oral Suspension
- Ofloxacin Oral Suspension
- Cephalexin Oral Suspension
- Coloxyl Oral Drops for Infants
- Transdermal Patch
- Antithyroid drugs
- Antacid Products
- Aluminium and Magnesium Trisilicate Tablets
- Aluminium Hydroxide Chewable Tablets
- Rabeprazole Tablets
- Ondansetron Injection
- Lansoprazole Capsules
- Esomeprazole Sodium Injection
- Omeprazole Injection
- Esomeprazole Domperidone Capsule
- Amoxicillin With Cloxacillin For Injection
- Pantoprazole injection
- Pantoprazole & Domperidone Capsules
- Antacid Capsules
- Ranitidine Tablets
- Pantoprazole Tablets
- Esomeprazole Tablets
- Esomeprazole Domperidone Capsules
- Rabeprazole Sodium Tablets
- Omeprazole Tablets
- Esomeprazole Magnesium Tablets
- Lansoprazole Delayed Release Capsules
- Ursodeoxycholic Acid Tablets
- Omeprazole Delayed Release Capsule
- Ranitidine Tablets
- Ranitidine Hydrochloride And Domperidone Tablets
- Esomeprazole Magnesium Tablets
- Loperamide Capsule
- Rabeprazole And Domperidone Capsules
- Compound Magnesium Tablets
- Pantoprazole Tablets
- Pantoprazole Sodium And Domperidone Capsule
- Ranitidine Tablets
- Rabeprazole Sodium Enteric coated Tablets
- Aluminium hydroxide Tablets
- Aluminium and Magnesium and Simethicone Chewable Tablets
- Aluminium and Magnesium and Simethicone Tablets
- Pharmaceutical Infusion
- Methocarbamol Injection
- Enoxaparin Sodium Injection
- Arsenic Trioxide Injection
- Methylcobalamin Injection
- Fosphenytoin Sodium Injection
- Alprostadil Injection
- Paracetamol Infusion
- Tobramycin Inhalation Solution
- Terbutaline Sulfate Injection
- Piroxicam Injection
- Ketorolac Trometamol Injection
- Mepivacaine Hydrochloride Injection
- Ofloxacin Infusion
- Pharmaceutical Injections
- Remdesivir Injection
- Granisetron Hydrochloride Injection
- Furosemide Injection
- Somavert Injection
- Atracurium Besylate Injection
- Milrinone Injection
- Cefazolin Sodium Injection
- Dexamethasone Sodium Phosphate Injection
- Dalteparin Sodium Injection
- Noradrenaline Birtartrate Injection
- Methylergometrine Maleate Injection
- Calcium Gluconate Injection
- Vitamin D3 Injection
- Protamine Sulphate Injection
- Glycopyrrolate Injection
- Fluconazole Injection
- Interferon Alfa Injection
- Leucovorin Calcium Injection
- Aceclofenac Injection
- Soluble Insulin Injection
- Carbetocin Injection
- Thiopental Sodium for Injection
- Vitamin K Injection
- Xylazine Injection
- Iron Dextran Injection
- Zinc Sulfate Injection
- Calcium + Vitamin B12 + Vitamin D3 Injection
- Gadobutrol Injection
- Mannitol Injection
- Cloprostenol Injection
- Iron Dextran Injection
- Adalimumab Injection
- Sodium Nitrite Injection
- Glutathione For Injection
- Human Normal Immunoglobulin Solution
- Fosphenytoin Sodium Injection
- Enrofloxacin injection
- Heparin Sodium Injection
- Fructose Sodium Diphosphate Injection
- Furosemide Injection
- Dimethyl Sulfoxide Irrigation
- Fosphenytoin Sodium Injection
- Enrofloxacin injection
- Ganciclovir for injection
- Ethamsylate Injection
- Epinephrine Injection
- Hydrocortisone Sodium Succinate for Injection
- Heparin Sodium Injection
- Haloperidol Decanoate Injection
- Enoxaparin Sodium Injection
- Ethamsylate Injection
- Glycopyrrolate Injection
- Granisetron Hydrochloride Injection
- Hydroxyzine Hydrochloride Injection
- Haloperidol Decanoate Injection
- Heparin Sodium Injection
- Haloperidol Injection
- Glycopyrrolate Injection
- Heparin Sodium Injection
- Fluconazole Injection
- Enoxaparin Sodium Injection
- Fraxiparine Injection
- Furosemide Injection
- Famotidine Injection
- Glucagon Injection
- Enoxaparin Sodium Injection
- Folic Acid Injection
- Hydroxocobalamin Injection
- Famotidine Injection
- Hydrocortisone Sodium Succinate for Injection
- Fluphenazine Decanoate Injection
- Enoxaparin Sodium Injection
- Etomidate Injection
- Hydroxocobalamin Injection
- Haloperidol Injection
- Heparin Sodium Injection
- Famotidine Injection
- Granisetron Hydrochloride Injection
- Folic Acid Injection
- Hydrocortisone Sodium Succinate for Injection
- Fraxiparine Injection
- Hydralazine Hydrochloride Injection
- Fructose Sodium Diphosphate Injection
- Haloperidol Decanoate Injection
- Glycopyrrolate Injection
- Hydrocortisone Sodium Succinate for Injection
- Hydroxyzine Hydrochloride Injection
- Enalaprilat Injection
- Furosemide Injection
- Fluorescein Injection
- Human Normal Immunoglobulin Solution
- Ethamsylate Injection
- Hydroxocobalamin Injection
- Filgrastim Injection
- Doxapram Hydrochloride Injection
- Drotaverine Injection
- Esmolol Hydrochloride Injection
- Edrophonium Chloride Injection
- Fraxiparine Injection
- Fluphenazine Decanoate Injection
- Glutathione For Injection
- Diclofenac Sodium Injection
- Diphenhydramine Hcl Injection
- Diltiazem Hydrochloride Injection
- Dantrolene Sodium Injection
- Citicoline Injection
- Dalteparin Sodium Injection
- Leuprolide Acetate Injection
- Cisatracurium Injection
- Citicoline Injection
- Cimetidine Injection
- Co-trimoxazole Concentrate Injection
- Diminazene Aceturate & Phenazone Injection
- Dalteparin Sodium Injection
- Diclofenac Sodium Injection
- Diphenhydramine Hcl Injection
- Dimercaprol Injection
- Desmopressin Acetate Injection
- Dexamethasone Sodium Phosphate Injection
- Vitamin B Complex Injection
- Cisatracurium Injection
- Dexamethasone Sodium Phosphate Injection
- Diltiazem Hydrochloride Injection
- Dicyclomine Hcl Injection
- Desmopressin Acetate Injection
- Dexamethasone Sodium Phosphate Injection
- Vitamin B Complex Injection
- Cyanocobalamin Injection
- Cocarboxylase Hydrochloride Injection
- Clonazepamum Injection
- Cimetidine Injection
- Dantrolene Sodium Injection
- Closantel Injection
- Diethylstilbestrol Injection
- Dalteparin Sodium Injection
- Dalteparin Sodium Injection
- Diltiazem Hydrochloride Injection
- Cholecalciferol Injection
- Dexamethasone Sodium Phosphate Injection
- Citicoline Injection
- Creatine Phosphate Sodium Injection
- Cysteine Hydrochloride Injection
- Dicyclomine HCL Injection
- Atenolol Injection
- Butorphanol Tartrate Injection
- Analgin Injection
- Bromhexine Hydrochloride Injection
- Bupivacaine HCL Injection
- Buparvaquone Injection
- Caffeine and Sodium Benzoate Injection
- carnitine injection
- Atracurium Besylate Injection
- Bupivacaine Hcl Injection
- Calcium Folinate Injection
- Analgin Injection
- Calcitriol Injection
- Aprotinin Injection
- Atropine Sulphate Injection
- Caffeine Citrate Injection
- Caspofungin Acetate For Injection
- Cefoperazone + Sulbactam For Injection
- Betamethasone Sodium Phosphate Injection
- Calcium Folinate Injection
- Calcium Chloride Injection
- Buparvaquone Injection
- Carboprost Tromethamine Injection 250
- B-Complex Liver Extract Injection
- Caspofungin Acetate For Injection
- Bovine Lipid Extract Surfactant Injection
- Cefazolin Sodium Injection
- Carboprost Tromethamine Injection
- Calcitriol Injection
- Atracurium Besylate Injection
- Calcitonin (Salmon) Injection
- Azathioprine For Injection
- Cefalexin Sodium For Injection
- Cefoperazone Injection
- Caffeine Citrate Injection
- Atosiban Injection
- Bupivacaine Injection
- Butorphanol Tartrate Injection
- B-Complex Injection
- Betamethasone and Diclofenac and Vitamin B12 Injection
- Cefepime for Injection
- Atropine Sulphate Injection
- Ascorbic Acid Injection
- Atracurium Besylate Injection
- Ascorbic Acid Injection
- Ascorbic Acid Injection
- Beractant Suspension for Injection
- Cefalexin Sodium For Injection
- Amoxicillin and Clavulanate Potassium Injection
- Amoxicillin and Clavulanate Potassium Injection
- Caffeine Citrate Injection
- Dimercaprol Injection
- Aminophylline Injection
- Amphotericin B Injection
- Papaverine Hydrochloride Injection
- Papaverine Hydrochloride Injection
- Aceclofenac Injection
- Atropine Sulphate Injection
- N Butyl Cyanoacrylate Injection
- Leuprolide Acetate Injection
- Aciclovir Intravenous Infusion Injection
- Folic Acid Injection
- Acetazolamide Injection
- Etofylline and Theophylline Injection
- Pantoprazole Sodium for injection
- Aciclovir Intravenous Infusion Injection
- Adalimumab Injection
- Tigecycline for Injection
- Procainamide Hydrochloride Injection
- Amiodarone Hydrochloride Injection
- Dihydralazine Mesilate for Injection
- Phytomenadione Injection
- Vecuronium Bromide For Injection
- Acetylcysteine Injection
- Acetylcysteine Injection
- Albumin Injection
- Adenosine Injection
- Amphotericin B Liposomal for Injection
- Amoxicillin Sodium With Cloxacillin Sodium For Injection
- Iron Sucrose Injection
- Amoxicillin Sodium and Clavulanate Potassium for Injection
- Adenosine Triphosphate Injection
- Sodium Nitroprusside Injection
- Alcuronium Chloride Injection
- Aflibercept Solution for Injection
- Aminocaproic Acid Injection
- Iron Sucrose Injection
- Amphotericin B Liposomal for Injection
- Vecuronium Bromide For Injection
- Iron Sucrose Injection
- Phytomenadione Injection
- Adrenaline injection
- Amifostine For Injection
- Procainamide Hydrochloride Injection
- Rocuronium Bromide Injection
- Aciclovir for Injection
- Adenosine Monophosphate Injection
- Aciclovir Intravenous Infusion Injection
- Adenosine Injection
- Protamine Sulphate Injection
- Neostigmine Injection
- Amiodarone Sterile Concentrate Injection
- Vecuronium Bromide For Injection
- Alfacalcidol Injection
- Neostigmine Injection
- Acetylcysteine Injection
- Aciclovir for Injection
- Amoxicillin Sodium with Salbactum Sodium for Injection
- Rocuronium Bromide Injection
- Procaine Hydrochloride Injection
- Rocuronium Bromide Injection
- Aminophylline Injection
- Vitamin D3 Injection
- Bupivacaine HCl. Injection
- Aggribloc Injection
- Rocuronium Bromide Injection
- Alpha Lipoic Acid injection
- Aminophylline Injection
- Sodium Acid Phosphate Granules Injection
- Meloxicam and Paracetamol Injection
- Methylergometrine Injection
- Heparin Sodium Injection
- Albumin Injection
- Sodium Chloride Injection
- Kyprolis Injection
- Tysabri Injection
- Acetylcysteine Injection
- Esmolol Injection
- Carboprost Injection
- Signifor Injection Pasireotide
- Mitomycin-C Injection
- Arsenic Trioxide Injection
- Metalyse Injection
- Netilmicin Injection
- Glycopyrrolate Injection
- Enoxaparin Injection
- Amiodarone Injection
- Tissue Plasminogen Activator Injection
- Visudyne Injection Verteporfin
- Nimodipine Injection
- Promethazine Injection
- Vitamin E Acetate Injection
- Zinc Sulfate Injection
- Xylazine Hcl Injection
- Vecuronium Bromide For Injection
- Voriconazole Injection
- Xylazine Injection
- Aminocaproic Acid Injection
- Aminophylline Injection
- Metoclopramide Injection
- Drotaverine Injection
- Vitamin A D3 E H Injection
- Zinc Sulfate Injection
- Hyoscine Butylbromide Injection
- Verapamil Hydrochloride Injection
- Vitamin K3 Injection
- Naloxone Injection
- Vecuronium Bromide For Injection
- Zoledronic Acid Injection
- Phenytoin Injection
- Furosemide Injection
- Acton Prolongatum Injection
- Corticotropin Injection
- Pralidoxime Iodide Injection
- Dexamethasone Injection
- Xylazine Hcl Injection
- Vitamin B Complex injection
- Vitamin Nicotinamide Injection
- Vitamin B6 Injection
- Topiramate Tablets
- Ceftazidime Injection
- Lignocaine Hcl Injection
- Hydralazine Injection
- Vitamin A Concentrate Injection
- Vitamin D3 Injection
- Verapamil Hydrochloride Injection
- Zoledronic Acid Injection
- Tranexamic Acid injection
- Valproate Sodium Injection
- Potassium Chloride for Injection
- Tranexamic Acid injection
- Piroxicam Injection
- Polidocanol Injection
- Piroxicam Injection
- Polidocanol Injection
- Ranitidine Injection
- Ropivacaine Hydrochloride Injection
- Tirofiban Hydrochloride Injection
- Sodium Bicarbonate Injection
- Theophylline + Etofylline Injection
- Ribavirin Injection
- Potassium Chloride for Injection
- Potassium Chloride for Injection Concentrate
- Ranitidine Injection
- Pralidoxime Chloride For Injection
- Triamcinolone Acetonide Injection
- Toldimfos Sodium Injection
- Tranexamic Acid injection
- Tranexamic Acid injection
- Tinzaparin Sodium Injection
- Succinylcholine Chloride Injection
- Tranexamic Acid injection
- Rabies Immunoglobulins Injection
- Triamcinolone Acetonide Injection
- Ropivacaine Hydrochloride Injection
- Tinzaparin Sodium Injection
- Toldimfos Sodium Injection
- Sodium Thiosulphate Injection
- Thiamine Injection
- Piroxicam Injection
- Ranitidine Injection
- Piroxicam Injection
- Thiamine Injection
- Tenoxicam For Injection
- Piroxicam and Paracetamol Injection
- Ranitidine Injection
- Thiopental Sodium for Injection
- Ropivacaine Hydrochloride Injection
- Theophylline + Etofylline Injection
- Thiamine Hydrochloride Injection
- Quinine Dihydrochloride Injection
- Tirofiban Hydrochloride Injection
- Rabies Immunoglobulins Injection
- Thiamine Hydrochloride Injection
- Sodium Chloride Injection
- Rabeprazole Sodium for Injection
- Sterile Water For Injection
- Succinylcholine Chloride Injection
- Pralidoxime Chloride For Injection
- Tirofiban Hydrochloride Injection
- Triamcinolone Acetonide Injection
- Soluble Insulin Injection
- Pyridoxine Hydrochloride Injection
- Sterile Water For Injection
- Succinylcholine Chloride Injection
- Polidocanol Injection
- Sodium Valproate Injection
- Thiocolchicoside injection
- Sodium Stibogluconate Injection
- Ranitidine Injection
- Succinylcholine Chloride Injection
- Sodium Nitrite Injection
- Rabies Immunoglobulins Injection
- Praziquantel Injection
- Thiocolchicoside Injection
- Pralidoxime Iodide Injection
- Salbutamol Injection
- Suxamethonium Chloride Injection
- Sodium Tetradecyl Sulphate Injection
- Sodium Valproate Injection
- Sterile Water For Injection
- Sodium Thiosulphate Injection
- Potassium Chloride for Injection Concentrate
- Ropivacaine Hydrochloride Injection
- Ribavirin Injection
- Levetiracetam Injection
- Multivitamin Injection
- Mepivacaine Hydrochloride Injection
- Labetalol Hydrochloride Injection
- Levetiracetam Injection
- Labetalol Hydrochloride Injection
- Methylcobalamin Injection
- Meloxicam Injection
- Methylprednisolone Sodium Succinate For Injection
- Menadione Sodium Bisulphite Injection
- Metoprolol Tartrate Injection
- Meloxicam Injection
- Piracetam Injection
- Metoclopramide Hydrochloride Injection
- Leucovorin Calcium Injection
- Isoprenaline Injection
- Metoclopramide Hydrochloride Injection
- Meglumine Gadoterate Injection
- Indocyanine Green for Injection
- Meglumine antimoniate injection
- Ketorolac Trometamol Injection
- Medroxyprogesterone Acetate Injection
- Methylprednisolone Sodium Succinate for Injection
- Mannitol Injection
- Methylprednisolone Acetate Injectable Suspension
- Labetalol Hydrochloride Injection
- Methylprednisolone Sodium Succinate For Injection
- Mepivacaine Hydrochloride Injection
- Magnesium Sulfate Injection
- Leucovorin Calcium Injection
- Multivitamin Injection
- Methylprednisolone Sodium Succinate For Injection
- Meglumine Gadoterate Injection
- Leucovorin Calcium Injection
- Methylergometrine Maleate Injection
- Metoprolol Injection
- Ibuprofen Injection
- Methylprednisolone Sodium Succinate For Injection
- Ibuprofen Injection
- Methylprednisolone Acetate Injectable Suspension
- Naloxone Injection
- Piracetam Injection
- Iodixanol Injection
- Magnesium Sulfate Injection
- Mesna Injection
- Hyoscine Butylbromide Injection
- Pamidronate Disodium Injection
- Hydroxyzine Hydrochloride Injection
- Iodixanol Injection
- Milrinone Injection
- Menadione Sodium Bisulphite Injection
- Magnesium Sulfate Injection
- Iohexol Injection
- Hyoscine Butylbromide Injection
- Piroxicam and Paracetamol Injection
- Iron Dextran Injection
- Mecobalamin Injection
- Mesna Injection
- Ibuprofen Injection
- Melonex Injection
- Methylprednisolone Acetate Injectable Suspension
- Mesna Injection
- Leucovorin Calcium Injection
- Methocarbamol Injection
- Methylcobalamin Injection
- Milrinone Lactate Injection
- Methylcobalamin Injection
- Ketorolac Trometamol Injection
- Pamidronate Disodium for Injection
- Hyoscine Butylbromide Injection
- Isophane Insulin Injection
- Mecobalamin Injection
- Meloxicam Injection
- Mepivacaine Hydrochloride Injection
- Methylprednisolone Acetate Injectable Suspension
- Iohexol Injection
- Drotaverine Hydrochloride Injection
- Furosemide Injection
- Etomidate Injection
- Micafungin Injection
- Isophane Insulin Injection
- Carnitine Injection
- Leucovorin Calcium Injection
- Magnesium Sulfate Injection
- Tinzaparin Sodium Injection
- Potassium Phosphate Injection
- Doxapram Hydrochloride Injection
- Essential Phospholipids Injection
- Filgrastim Injection
- Suxamethonium Chloride Injection
- Hydroxyzine Hydrochloride Injection
- Alprostadil Injection
- Bulk Drugs
- Rapid Diagnostic Kit
- Medicated Soap
- Medicated Hair Oil
- Energy & Power Enhancer
- Anticancer Injection
- Leucovorin Calcium Injection
- Cytarabine Injection
- Daunorubicin Injection
- Emgrast Injection
- Aldesleukin Injection
- Methotrexate Injection
- Teniposide Injection
- Fulvestrant Injection
- Methotrexate Injection
- Epirubicin Hydrochloride Injection
- Thalidomide Capsules
- Dactinomycin for Injection
- Dacarbazine For Injection
- Pemetrexed Disodium For Injection
- Doxorubicin Hydrochloride Injection
- Paclitaxel Injection
- Carboplatin Injection
- Carboplatin Injection
- Carboplatin Injection
- Trastuzumab For Injection
- Irinotecan Hydrochloride Injection
- Irinotecan Hydrochloride Injection
- Trastuzumab For Injection
- Methotrexate Injection
- Pemetrexed Disodium For Injection
- Fluorouracil Injection
- Doxorubicin Hydrochloride Injection
- Doxorubicin Hydrochloride Injection
- Fluorouracil Injection
- Azacitidine For Injection
- Paclitaxel Injection
- Mitomycin for Injection
- Fludarabine Phosphate Injection
- Methotrexate Injection
- Vinblastine Sulphate Injection
- Vinorelbine Injection
- Idarubicin Hydrochloride for Injection
- Yondelis Injection
- Bleomycin Sulphate For Injection
- Mitoxantrone Injection
- Cisplatin Injection
- Cisplatin Injection
- Cladribine Injection
- Docetaxel Injection
- Gemcitabine for Injection
- Docetaxel Injection
- Mitoxantrone Injection
- Docetaxel Injection
- Etoposide Injection
- Mitoxantrone Injection
- Irinotecan Hydrochloride Injection
- Rituximab Injection
- L-Asparaginase Injection
- L-Asparaginase Injection
- Leuprolide Acetate Injection
- Methotrexate For Injection
- Pertuzumab Injection
- Mesna and Ifosfamide Injection
- Melphalan Injection
- Mesna and Ifosfamide Injection
- Mitomycin for Injection
- Mitomycin for Injection
- Mitomycin for Injection
- L-Asparaginase Injection
- Thiotepa for Injection
- Bleomycin for Injection
- Idarubicin Hydrochloride for Injection
- Vincristine Sulfate Injection
- Idarubicin Hydrochloride for Injection
- Mitomycin for Injection
- Amsacrine Injection
- Mechlorethamine Hcl for Injection
- Mechlorethamine Hcl for Injection
- Thiotepa for Injection
- Emgrast M Injection
- Oxaliplatin Injection
- Gemcitabine for Injection
- Gemcitabine for Injection
- Vincristine Sulfate Injection
- Cyclophosphamide For Injection
- Vinblastine Sulphate Injection
- Bendamustine Hydrochloride Injection
- Bendamustine injection
- Cyclophosphamide Injection
- Cytarabine Injection
- cytarabine injection
- Docetaxel Injection
- cytarabine injection
- Dacarbazine For Injection
- Dacarbazine For Injection
- Bevacizumab Injection
- Bevacizumab Injection
- Cabazitaxel Injection
- Cisplatin Injection
- Rituximab Injection
- Epirubicin Hydrochloride for injection
- Ifosfamide for Injection
- Paclitaxel Injection
- Dactinomycin for Injection
- Paclitaxel Injection
- cytarabine injection
- Cytarabine Injection
- Decitabine For Injection
- Paclitaxel Injection
- cytarabine injection
- Cytarabine Injection
- Ifosfamide for Injection
- Decitabine For Injection
- Cabazitaxel Injection
- Epirubicin Hydrochloride Injection
- Paclitaxel Injection
- Carmustine For Injection
- Cisplatin Injection
- Cyclophosphamide For Injection
- Fluorouracil Injection
- Methotrexate Injection
- Epirubicin Hydrochloride for injection
- Doxorubicin Hydrochloride Injection
- Methotrexate Injection
- Vinorelbine Injection
- Docetaxel Injection
- Bortezomib Injection
- Zolendronic Acid for Injection
- Pemetrexed Injection
- Gemcitabine Injection
- Cisplastin Injection
- RITUXIMAB INJECTION
- Pemnat Injection
- Paclitaxel Injection
- Bleomycin Injection
- Doxorubicin Hydrochloride Injection
- Etoposide Injection
- Cisplastin Injection
- Carboplatin Injection
- Ondansetron Injection
- Epirubicin Hydrochloride Injection
- Granisetron Injection
- Fluorouracil Injection
- Tamoxifen Tablets
- Erythropoietin Injection
- Bleomycin Injection 15 units
- Anti Cancer Tablet
- Lomustine Capsules
- Procarbazine Capsule
- Prostate Cancer Medicines
- Lung cancer medicine
- Erlotinib Tablets
- Erlotinib Tablets
- Capecitabine Tablets
- Methotrexate Tablets
- Hydroxyurea Capsules
- Busulphalan Tablets
- Capecitabine Tablets
- Sorafenib Tablets
- Curcumin Tablets
- Abiraterone Acetate Tablets
- Bicalutamide Tablets
- Crizonix Capsules
- Estramustine Capsules
- Capecitabine Tablets
- Etoposide Capsules
- Dexamethasone Tablets
- Methotrexate Tablets
- Methotrexate Tablets
- Etoposide Tablets
- Everolimus Tablets
- Hydroxyurea Capsules
- Lenalidomide Capsules
- Melphalan Tablets
- Mycophenolate mofetil Tablets
- Sirolimus Tablets
- Abiraterone Acetate Tablets
- Anastrozole Tablets
- Capecitabine Tablets
- Mesalamine Tablet
- Anti cancer Tablets
- Cyclophosphamide Tablets
- Cyclophosphamide Tablets
- Cyclophosphamide Tablets
- Mercaptopurine Tablets
- Etoposide Capsules
- Flutamide Capsules
- Lenalidomide Capsule
- Lenalidomide Capsule
- Procarbazine Hydrochloride Capsule
- Temozolomide Capsules
- Mitotane Tablets
- Everolimus Tablet
- Anti cancer Tablet
- Anastrazole Tablets
- Dasatinib Tablets
- Dasatinib Tablets
- Hydroxyurea Capsules
- Mercaptopurine Tablets
- Tamoxifen Tablet
- Exemestane Tablets
- Crizotinib capsule
- Hydroxyurea Capsules
- Temozolomide Capsules
- Bortenat Injection
- Erlotinib Tablets
- Gefitinib Tablet
- Etoposide Capsules
- Finasteride Tablets
- Flutamide Tablets
- Finasteride Tablets
- Tamoxifen Citrate Tablets
- Temozolomide Capsules
- Temozolomide Capsules
- Melphalan Tablets
- Tacrolimus Capsules
- Tacrolimus Capsules
- Methotraxate Tablets
- Temozolomide Capsules
- Tamoxifen Citrate Tablets
- Mycophenolate Sodium Tablets
- Etoposide Tablets
- Gefitinib Tablets
- Ibandronic Acid Injection
- Estramustine Phosphate Capsules
- Bicalutamide Tablets
- Abiraterone Tablets
- Flutamide Tablets
- Cyclophosphamide Tablets
- Cyproterone Tablets
- Anastrozole Tablets
- Asthma & Tuberculosis Products
- Ethambutol Tablets
- Ethambutol with Isoniazid Tablets
- Rifampicin Isoniazid Pyrizinamide Tablets
- Rifampicin Isoniazid Tablets
- Pyrazinamide Tablets
- Cycloserin Capsules
- Rifampicin Isoniazid Pyrazinamide With Ethambutol Tablets
- Rifampicin Capsules
- Isoniazid Capsules
- Prothionamide Tablets
- Ethionamide Tablets
- Isoniazid Tablets
- Anti Allergic Tablets
- Tenofovir disproxil tablets
- Desloratadine Tablets
- Fexofenidine HCL Tablets
- Cetrizine Hydrochloride Tablets
- Chlorpheniramine Tablets
- Levocetrizine Hydrochloride Tablet
- Levocetrizine hydrochloride & Montelukast Tablets
- Cetirizine hydrochloride Tablets
- Montelukast Tablets
- Fexofenidine HCL Tablets
- Combination of Paracetamol Tablets
- Antiviral Medicines
- Hepatitis B medicines
- Ayurvedic Product
- Herbal Tablets
- Guggulu Tablets
- Guduchi Tablets
- Rumalaya Tablets
- Septilin Tablets
- Herbal Painkiller Capsule
- Liv 52 Tablets
- Bael Tablets
- Shatavari Tablets
- Diabecon Tablets
- Geriforte Tablets
- Karela Tablets
- Carica Papaya Leaf Extract Tablets
- Abana Tablets
- Serpina Tablets
- Bresol Tablets
- Hadjod Tablets
- Tagara Tablets
- Pilex Tablets
- Brahmi Tablets
- Shallaki Tablets
- Cystone Tablets
- Lukol Tablets
- Himalaya Speman Tablets
- Gokshura Tablets
- Seeds
- Pharmaceuticalcream And Gel
- Diclofenac Methylsalicilate Menthol Linseed Oil Gel
- Diclofenac Gel
- Hydrocortisone Acetate Cream
- Ketoconazole Pyrithione Zinc Lotion
- Hydroquinone Cream
- Heparin Sodium Gel
- Clotrimazole Cream
- Betamethasone and Salicylic Acid Ointment
- Betamethasone and Fusidic Acid Cream
- Anti Heamorrhoid Ointment
- Clindamycin Phosphate Gel
- Cetrimide Cream
- Clotrimazole Beclomethasone Dipropionet and Neomycin Sulfate Cream
- Cold Cream
- Hydroquinone Cream
- Erythromycin Gel
- Magnesium Sulfate Paste
- Clotrimazole Gentamycin Beclomethasone Dipropionate Cream
- Desonide Cream
- Gamma Banzene Hexachloride Proflavine Hemisulfate Cetrimide Cream
- Betamethasone Valerate Ointment
- Bacitracine Ointment
- Betamethasone Dipropionate Clotrimazole Neomycin Sulfate Cream
- Benzoyl Peroxide Cream
- Aciclovir Cream
- Betamethasone and Salicylic Acid Cream
- Menthol and Aqueous Cream
- Beclometasone Dipropionate and Clotrimazole Cream
- Clindamycin Phosphate and Benzyl Peroxide Gel
- Aciclovir Eye Ointment
- Chloromycetin Eye Ointment
- Bacitracine Cream
- Clobetasol Propionate Cream
- Betamethasone Cream
- Betamethasone and Salicylic Acid Ointment
- Chlorhexidine Obstetric Cream
- Acyclovir Ointment
- Betamethasone Clioquinol Cream
- Tretinoin Gel
- Joen Fruit Skin Cream
- Ichthammol Ointment
- Adapheline Gel
- Hair Gel (Aragan Oil)
- Nitrofurazone Cream
- Clobetasol propionate + Miconazole Nitrate Cream
- Hydrocortisone Cream
- Tazarotene Gel
- Tetracycline Hydrochloride Ointment
- Gentamicin Gel
- Imiquimod Cream
- Vaseline jelly
- Aqueous Calamine Cream
- Anti Heamorrhoidal Cream
- Permethrin Cream
- Triamcinolone Acetonide Cream
- Sulfacetamide Sodium Ophthalmic Ointment
- Ciprofloxacin Eye Cream
- Atropine Eye Ointment
- Zinc Oxide Ointment
- Neomycin Sulfate and Bacitracin Ointment
- Benzoyl Peroxide Gel
- Zinc Oxide Cream
- Dinoprostone Gel
- Diclofenac Sodium Gel
- Clobetasol Propionate Ointment
- Cetrimide and Chlorhexidine Cream
- White Soft Paraffin Jelly
- Neomycin Eye Ointment
- Azithromycin Eye Ointment
- Miconazole Nitrate Cream
- White Soft Paraffin and Light Liquid Paraffin Cream
- Ciprofloxacin Eye Ointment
- Ofloxacin + Ornidazole + Clobetasol Propionate + Terbinafine Hydrochloride Cream
- Silver Sulfadiazine with Chlorhexidine Gluconate Cream
- Salicylic Acid Ointment
- Silver Sulfadiazine Cream
- Clobetasol propionate + Salicylic Acid Ointment
- Clotrimazole Beclomethasone Dipropionet Cream
- Chlortetracycline Eye Ointment
- Compound Benzoic Acid Ointment
- Tretinoin Cream
- Methylprednisolone Cream
- Tretinoin Cream
- Clotrimazole + Gentamycin + Beclomethasone Dipropionate cream
- B-Sitosterol Ointment
- Salicylic Acid Ointment
- Benzoyl Peroxide Gel
- Methyl Salicylate Ointment
- Neomycin Sulfate and Bacitracin Ointment
- Tazarotene Gel
- Povidone-Iodine Ointment
- Nimesulide Gel
- Povidone-Iodine Ointment
- Prednisolone Cream
- Silicon Dioxide Gel
- Bacitracin Zinc Ointment
- Terbinafine HCL Cream
- Tetracycline Hydrochloride Ointment
- Nystatin Ointment
- Permethrin Cream
- Nystatin Cream
- Pimecrolimus Cream
- Nitrofurazone Ointment
- Econazole Nitrate Cream
- Diclofenac Sodium Gel
- Ciclopirox Olamine Cream
- Benzoic and Salicylic Acids Ointment
- Bacitracin Ointment
- Calcipotriol Ointment
- Dinoprostone Gel
- Clotrimazole Cream
- Clotrimazole Cream
- Aqueous Cream
- Methylprednisolone Ointment
- Gentamicin Sulfate Cream
- Zinc Oxide Cream
- Podophylline Cream
- Benzoyl Peroxide Cream
- Thiabendazole Ointment
- Aceclofenac Gel
- Betamethasone Gentamycin Cream
- Hydrocortisone Ointment
- Moov Cream
- Canesten Topical Antifungal Cream
- Sliver Sulfadazine with Chlorhexidine Cream
- Mepyramine Maleate Cream
- Sertaconazole Nitrate Cream
- Mometasone Furoate Cream
- Fusidic Acid With Betamethasone Cream
- Fluorouracil Cream
- Tazarotene Cream
- Triamcinolone Acetonide Oral Paste
- Zinc oxide Ointment
- Lignocaine Hydrochloride Gel
- Hydrocortisone Cream
- Clotrimazole Tube
- Hemorrhoidal Cream
- Amorolfine Cream
- Clotrimazole Creams
- Burn Cream
- Fluocinolone Acetonide Cream
- Diclofenac Gel
- Antiseptic Cream
- Diclofenac Sodium Gel
- Clobetasol Cream
- Pharmaceutical cream
- Retino A Cream
- Gentamycin Cream
- Fluticasone Propionate Cream
- Neomycin Sulfate Cream
- Sisomicin Sulfate Cream
- Diclofenac Methylsalicilate Menthol Linseed Oil Gel
- Tretinoin + Clindamycin Gel
- Nystatin Ointment
- Sucralafate Ointment
- Nystatin Ointment
- Miconazole + Flucinolone acetonide Ointment
- Betamethasone + Cliquinol Cream
- Silver Sulfadiazine cream
- Neomycin, Polymyxin B Sulfate and Bacitracin Ointment
- Azithromycin Adaphalene Aloe Vera cream/Gel
- Permethrin Cream
- Mometaosone Furorate Ointment
- Betamethasone , Miconazole, Gentamycin Sulphate Cream
- Salicylic Acid + Mometasone Ointment
- Vicks Vaporub
- Sulfur Ointment
- Tacrolimus Ointment
- Hydroquinone Gel
- Neomycin and Bacitracin Ointment
- Mucopolysaccharide Gel
- Betamethasone Clotrimazole Neomycin Sulphate Cream
- Soframycin Cream
- Penciclovir Cream
- Metronidazole + Chlorhexidine Gluconate Sodium Gel
- Moisturizing cream
- Diclofenac Gel
- Fusidic acid Cream
- Hydrocortisone Cream
- Tacrolimus Ointment
- Tretinoin Gel
- Metronidazole Gel
- Clobetasol Ointment
- Chlorhexidine Gluconate
- Absolute Alcohol Gel
- Clindamycin Gel
- Ketoconazole Cream
- Betamethasone Valerate Cream
- Dimethindene Gel
- Hydrocortisone Ointment
- Mupirocin Ointment
- Ketoprofen Gel
- Heparin Sodium Gel
- Sodium Fusidate Ointment
- Benzyl Peroxide
- Coal Tar Salicylic acid Ointment
- Mometaosone Furorate Cream
- Aciclovir Cream
- Neomycin Gel
- Salicylic acid Ointment
- Clobetasol Cream
- Vicks Inhaler
- Clobetasol Miconazole Gentamycin Sulphate Cream
- Fusidic Acid + Betamethasone Cream
- Vicks Vaporub
- Alprostadil Cream
- Fluconazole Mouth Paint
- Methyl Salicylate Menthol Camphor Cream
- Azithromycin Gel
- Hydrocortisone Cream
- Ketoprofen Gel
- Fluocinolone Acetonide & Ciclopirox Olamine Cream
- Veterinary Injections
- Carbon & Chemicals
- Solvent
- Active Pharmaceutical Ingredients
- Albuterol Sulphate
- Acrivastine
- Formoterol Fumarate Dihydrate
- Agomelatine
- Belinostat
- Attapulgite
- Metformin
- Tenofovir Alafenamide Fumarate
- Tamsulosin
- Amikacin Sulphate
- Voglibose
- Sulbactam Sodium Sterile
- Efavirenz
- Atazanavir
- Emtricitabine
- Multivitamin Pellets
- Sertraline hcl
- Procyclidine
- Cephradine
- D Sorbitol
- Clopidogrel Bisulphate
- Ofloxacin
- Azithromycin
- Itraconazole
- Fexofenadine
- Methyldopa Anhydrous
- Racecadotrill
- Domperidone
- Ciprofloxacin
- Fexofenadine hcl
- Palonosteron hcl
- Tenofovir Disproxil Fumarate
- Glucosamine Sulphate
- Amlodipine Besylate
- Telmisartan
- Xylometazoline Hydrochloride
- Ampicillin BP Compacted
- Solifenacin Succinate
- Esomeprazole Pellets
- Gefitinib
- Naproxen Sodium
- Pioglitazone
- Thiocolchicoside
- Rabeprazole Sodium
- Acyclovir
- Calcitriol
- Metronidazole
- Desloratadine
- Voriconazole
- Ranitidine Hydrochloride
- Hydrochlorothiazide
- Atazanavir Sulphate
- Topiramate
- Aripiprazole
- Loratidine
- Paracetamol
- Ivermectin
- Carvedilol
- CEPHRADIN Cephradine Anhydrous
- Albendazole Api
- Cabergoline
- Pregabaline
- Active Pharmaceutical Ingredients
- PVP K30
- Piperazine Adipate
- Tulobuterol Base
- Salmeterol Xinafoate
- Prasugrel Api
- Vitamin C Coated
- PVP K12
- PVP K60
- Moxifloxacin hcl
- VORICONAZOLE API
- Salbutamol Base
- Levalbuterol Tartrate
- Oxcarbazepine
- Fingolimod Api
- Aminocaproic Acid
- Ondansetron Hcl
- Ivabradine
- Tulobuterol Hcl
- Fenoterol Hydrobromide
- Ipratropium Bromide
- Adapalene Api
- Everolimus
- Acetretin
- Chymotrypsin
- Capecitabine
- Paclitaxel
- Docetaxel
- Nirmatrelvir Active pharma
- Pharmaceutical Intermediate
- Speciality Fine Chemical
- Acetone Sodium Bisulfite
- Acetamide 98%
- Acetobromo-D-Glucose 98%
- 2-Acetonaphthone 98%
- ABTS extrapure
- Aebsf Hydrochloride Extrapure Ar
- Abscisic Acid
- ABTS Substrate Solution
- PUGNAc extrapure, 95%
- Acetamido Tempo
- Acarbose 95%
- ABTS Peroxidase Stop Solution
- ACES Buffer Powder
- ACES Buffer Powder
- 3-(Acetamidomethylthio)propanoic Acid
- 1-Acetonaphthone 98%
- Acetobromo-D-Galatose 98%
- Extended or Sustained Release Pellets
- Eye & Ear & Nasal Drops
- Apraclonidine Hydrochloride Eye Drops
- Fluconazole Eye Drops
- Aerosol Eye drops
- Moxyfloxacin Predenisolone Eye Drops
- Fluticasone Nasal Spray
- Norfloxacin Eye/Ear Drops
- Olopatadine Hydrochloride Ophthalmic Solution Eye Drops
- Moxifloxacin Ophthalmic Solution Eye Drops
- Moxifloxacin and Ketorolac Tromethamine Ophthalmic Solution Eye Drops
- Naphazoline HCL and Chlorpheniramine Maleate Eye Drop
- Moxifloxacin and Prednisolone Acetate Eye Drops
- Dorzolamide Hcl Eye Drops
- Timolol Maleate Eye Drops
- Saline Nasal Solution Spray
- Ofloxacin & Dexamethasone Eye/Ear Drops
- Neomycin Sulfate and Dexamethasone Sodium Phosphate Ophthalmic Solution Eye Drops
- Antazoline HCL and Tetrahydrozoline HCL Eye Drop
- Fluorescein Eye drops
- Moxifloxacin Eye Drops
- Norfloxacin eye drops
- Balanced Salt Solution Eye Drops
- Flurbiprofen Eye Drops
- Clotrimazole + Lignocaine HCL Ear Drops
- Dorzolamide Hydrochloride and Timolol Eye Drops
- Latanoprost Ophthalmic Solution Eye Drops
- Brimonidine Tartrate Eye Drops
- Fluorescein Injection
- Fluticasone Propionate Nasal Drops
- Cyclopentolate Eye Drop
- Sulfacetamide Sodium Eye Drops
- Carteolol Hcl Eye Drops
- Sulfacetamide Eye Drops
- Xylometazoline HCL Nasal Solution
- Dexamethasone Sodium Phosphate Eye Drop
- Sodium Chloride Eye Drops
- Salbutamol Respirator Solution
- Salbutamol Nebuliser Solution
- Xylometazoline Nasal Drops
- Levofloxacin Eye Drops
- Dexamethasone Sodium Phosphate Eye Drop
- Sodium Chloride Nasal Drops
- Ciprofloxacin and Dexamethasone Ear Drops
- Fusidic Acid Viscous Eye Drops
- Atropine Eye Drops
- Brimonidine and Timolol Eye Drops
- Ciprofloxacin and Dexamethasone Eye Drops
- Sodium Bicarbonate Ear Drops
- Antazoline Hydrochloride and Tetryzoline Hydrochloride Eye Drops
- Brimonidine Tartrate Eye Drops
- Diclofenac Sodium Eye Drops
- Ketorolac Tramthamine Eye Drops
- Ketotifen Fumarate Eye Drops
- Tetracaine Hydrochloride Eye Drop
- Latanoprost and Timolol Maleate Eye Drop
- Ciprofloxacin Eye Drop
- Latanoprost Eye Drop
- Timolol Eye Drops
- Fluticasone Nasal Spray
- Olopatadine Hydrochloride Ophthalmic Solution Eye Drops
- Moxifloxacin and Ketorolac Tromethamine Ophthalmic Solution Eye Drops
- Moxifloxacin and Dexamethasone Eye Drops
- Ciprofloxacin + Hydrocortisone Ear Drops
- Indometacin Eye Drops
- Saline Nasal Drops
- Zinc Sulphate Eye Drops
- Betamethasone Eye Drops
- Tetracaine Hydrochloride Eye Drop
- Fluorometholone Eye Drops
- Salbutamol Nebuliser Solution
- Travoprost and Timolol Maleate Opthalmic Solution Eye Drops
- Dexamethasone Sodium Phosphate Ophthalmic Solution Eye Drops
- Rifamycin Sodium Eye Drop
- Moxifloxacin Dexamethasone Eye Drops
- Ofloxacin ophthalmic solution Eye Drops
- Boric Acid Ear Drops
- Ciprofloxacin Eye Drops
- Ketorolac Tromethamine Eye Drops
- Betaxolol Eye Drops
- Neomycin Sulfate and Dexamethasone Sodium Phosphate Ophthalmic Solution Eye Drops
- Travoprost Ophthalmic Solution Eye Drops
- Triamcinolone Acetonide Nasal Spray
- Brimonidine Tartrate and Timolol Maleate Eye Drop
- Cyclopentolate Ophthalmic Solution Eye Drops
- Ipratropium Bromide Nasal Spray
- Beclomethasone Dipropionate, Ofloxacin, Clotrimazole and Lignocaine Hydrochloride Ear drops
- Tropicamide Eye Drops
- Dexamethasone and Neomycin Sulfate Eye Ear Drops
- Atropine Sulphate Eye Drops
- Rifampicin Eye drops
- Hypromellose Ophthalmic Solution Eye Drop
- Ciprofloxacin and Dexamethasone Eye/Ear Drops
- Sodium Cromoglicate Eye Drop
- Homatropine HCL Eye Drop
- Bimatoprost Eye Drops
- Timolol Maleate Ophthalmic Eye Drops
- Brimonidine and Timolol Eye Drops
- Amikacin Eye Drops
- Benzalkonium Chloride Eye Drop
- Sodium Chloride Eye Drop
- Timolol Maleate Eye Drops
- Desmopressin Nasal Spray
- Dorzolamide Hydrochloride Eye Drops
- Tropicamide & Phenylephrine Eye Drops
- Fluorometholone Eye Drops
- Flomist Nasal Spray
- Brimonidine Tartarate Eye Drops
- Olopatadine Eye Drops
- Clotrimazole Lignocaine Ear Drops
- Clotrimazole Chloramphenicaol Ear Drops
- Fluticasone Furoate Nasal Spray
- Ofloxacin Ketorolac Eye Drops
- Gentamycin Dexamethasone Eye Drops
- Cineraria Maritima Eye Drops
- Moxifloxacin loteprednol Eye Drops
- Cyclopentolate Eye Drops
- Ofloxacin Eye Drops
- Ofloxacin Clotrimazole Beclomethasone Ear Drops
- Xylometazoline hydrochloride nasal solution
- Atropine sulphate Eye Drops
- Olive Oil Ear Drops
- Moxifloxacin Ketorolac Eye Drops
- Pottasium iodide sodium chloride calcium iodide eye drops
- Ciprofloxacin Ear Drops
- CMC Gel Eye Drops
- Nepafenac Eye Drops
- Tobramycin with Dexamethasone Eye Drops
- Saline Nasal Drops
- Sodium Bicarbonate Ear Drops
- Cyclopentolate Phenylephrine Eye Dros
- Furamist Nasal Sprays
- Tobramycin Eye Drops
- Cyclosporine Eye Drops
- Oxymetazoline HCL Nasal Drops
- Isotonic Nasal Drops
- Amikacin Eye Drops
- Olopatadine Eye Drops
- Tropicamide Phenylephrine Eye Drops
- Azithromycin Eye Drops
- Sodium Chloride Eye Drops
- Duonase Nasal Spray
- Budesonide Aqueous Nasal Spray
- Olopatadine Eye Drops
- Travopost Eye Drops
- Betamethasone Eye Drops
- Latanoprost Eye Drops
- Atropine Sulphate Eye Drops
- Betaxolol Eye Drops
- Brimonidine Timolol Eye Drops
- Refersh Tears Eye Drops
- Tropicamide Ophthalmic Solution
- Tropicamide Ophthalmic Solution
- Sodium Carboxymethyl Cellulose Eye Drops
- Moxifloxacin Eye Drops
- Bimatoprost Eye Drops
- Dorzolamide Timolol Eye Drops
- Sodium Bicaronate Ear Drops 10ml
- Fluconazole Eye Drops
- Mometasone Furoate Nasal Spray
- Sodium Cromoglycate Eye Drops
- Dorzolamide Eye Drops
- Diclofenac Eye Drops
- Ciprofloxacin Eye Drops
- Moxifloxacin Dexamethasone Eye Drops
- Ofloxacin Dexamethasone Eye Drops
- Surgical Product
- Surgical Sutures
- Gigli saw handle
- Masking Tape
- Disposable Needle
- Surgical Gloves
- Three Way Stop Cock
- Disposable Syringes
- Condom Extra Sensitive
- Surgical Cap
- Measured Volume Set
- Central Venous Catheter
- Premium Adult Diapers
- Manual Resuscitator
- Toomey Syringe
- Surgical Blades
- Crescent knives
- Gigli saw wire
- Jet Nebulizer Chamber
- Endotracheal Tube Cuffed E.T Tube
- Urethral Catheter
- Scalp Vein Set
- Nasojejunal Feeding Tube
- Endotracheal Tube Intubation Stylet
- Blood Administration Set
- ReInforced Endotracheal Tube
- Kehrs T Tube
- Nasopharyngeal Airway
- Closed Wound Suction Unit
- Cvp Manometer
- Foley Catheter Silicone
- Nebulizer Chamber
- Closed Suction System
- Endo Bronchial Suction Catheter
- Intra Venous Cannula
- Micro Drop Infusion Set With Flow Rate Controller
- Polyurethane Feeding Tube
- Premium Infusion Set
- Nebulizer Chamber Power Drool
- Urine Bag With Measured Volume Chamber
- Male External Catheter
- Chest Drainage Catheter
- Needle Free Extension Line
- Intra Venous Infusion Kit
- Colostomy Bag
- Intra Venous Cannula Intraflon
- Bain Circuit
- Endotracheal Tube
- Infusion Bottle Adaptor
- Urine Collecting Bag With Bottom Outlet
- Spinal Needle
- Percutaneous Tracheostomy Tube
- HME Filter
- Abdominal Drainage Kit
- Laryngeal Mask Airways
- Foley Catheter
- Closed Wound Suction Unit
- High Pressure Monitoring Line
- Keratome Blades
- Iv Infusion Set
- Support Belt
- Surgical Disposabel Face Mask
- Silicone Foley Catheter
- Urine Bags
- Gauze Swab
- Cotton Wool Ball
- Soluset Iv Set With Dosage Burette
- MVR Blades
- Pap Smear Kit
- Conventional Syringe
- Ethylene Oxide Gas Indicator Tape
- Haematuria Foley Catheter
- Thoracic Catheter
- Double J Stent
- Hydrocephalus Shunt System
- Epidural Anesthesia Kit
- Blood Transfusion Kit
- Trachea Tee Plus
- Micro Drip Infusion Set
- Tracheostomy Tube Cuffed
- Pedia Drip
- Paedaitric Urine Collecting Bag
- Gas Indicator Tape
- Insulin Syringes
- Pen Needles
- X Ray Detectable Gauze
- Three Way Catheter
- Medical Adhesive Tape
- Micropore Paper Tape
- Masking Tape
- Disposabe Vaginal Speculum
- Surgical Skin Marker Pen
- Transparent IV Dressing
- Disposable Underpads
- Bone wax
- Paper Tape
- Intra Venous Dressing
- Absorbable Gelatin Sponge
- Nanofiber Mask
- Nitrile Gloves
- Disposable Razor
- Thoracic Drainage Catheter
- Umbilical Cord Clamp
- Surgeon Gowns
- Thoracic Drainage Catheter
- Hypodermic Syringe
- Adult Diapers
- IV Dressing
- AV Fistula Needels
- Paediatric IV Cannula Micron
- Yankaur Suction Set
- Jackson Pratt Drainage Set
- Endobronchial Tube
- Multi Gang Manifold
- Vented Infusion Set with Micron Filter
- Oxygen Mask
- Corrugated Drainage Sheet
- Twin Bore Oxygen Cannula
- Tracheostomy Tube
- Oxygen Mask
- Asepto Pump
- Male Extrenal Catheter
- Ventilator Circuit
- Precision Controller with Extension Set
- Intra Venous Cannula with 3 Way Stopper
- Catheter Mount
- Hi Line Spiral
- Enteral Feeding Bag
- Heavy Duty Pressure Fusor Cuff
- Paediatric Intra Venous Cannula
- Infusion Set
- Instant Flashback IV Cannula
- Under Water Seal Bottle with Valve
- Safety Intra Venous Cannula
- Percutaneous Tracheostomy Kit
- Ryles Tube
- Transurethral Resection Set
- Iv Cannula With Injection Port
- Supra pubic Balloon Catheter With Trocar
- Flower Tip Suction Catheter
- Large Drip Chamber With Air Stop Filter
- High Concentration Oxygen Mask
- Laryngeal Mask Airways
- Micro Drop Infusion Set
- Infant Feeding Tube
- Guedel Airways
- Buretta
- Hospital & Medical Supplies
- Pain Relief Hot And Cold Pack
- Hand Sanitizer
- Aneroid Sphygomanometer
- Digital Blood Pressure Monitor
- BP Apparatus
- BCG Syringe
- Blood Collection Bag
- Autoclave Indicator Tape
- Blood Transfusion Set
- Gauze Roll
- Interchangeable Glass Syringe
- Digital Thermometer with Flexibel Shaft
- Finger Tip Pulse Oximeter
- ECG Electrodes
- Paediatric Nebulizer
- Nebulizer with Piston Pump
- Mucus Extractor
- Nebulizer Aerosol Therapy
- Respiro Meter
- Pain Relief Hot And Cold Pack
- Bed Sore Prevention Kit
- Bed Sore Prevention Kit Cellmat
- Nebulizer Machine
- Eye Reusable Gel Pack
- Gluco Meter
- Sphygmomanometer Mercury Free
- Sterile Surgeon Gloves
- Cardiac Product
- Acebutolol Tablets
- Milrinone Injection
- Alteplase For Injection
- Amlodipine with Telmisartan Tablet
- Bumetanide Injection
- Pravastatin Tablets
- Cilnidipine Tablets
- GTN Transdermal Patch
- Streptokinase Injections
- Dobutamine Injection
- Dobutamine Injection
- Pentosan Polysulfate Sodium Capsule
- Digoxin Injection
- Digoxin Injection
- Digoxin Injection
- Bumetanide Injection
- Diazoxide Capsule
- Midodrine Hydrochloride Tablets
- Sodium Nitroprusside Injection
- Acenocoumarol Tablets
- Mephentermine Injections
- Labetalol Injection
- Apixaban Tablets
- Adenosine Injection
- Acenocoumarol Tablets
- Dipyridamole Injection
- Streptokinase Injection
- Rosuvastatin Tablet
- Lovastatin Tablets
- Aprotinin Injections
- Acenocoumarol Tablets
- Alteplase For Injection
- Fluvastatin Tablets
- Urokinase Injection
- Sublingual Tablets
- Aztor Tablet
- Micardis Tablets
- Telmisartan Tablet
- Doburamine Injections
- Ranolazine Tablets
- Losartan Potassium Tablets
- Losartan Tablets
- Tenecteplase Injection
- Tenecteplase Injection
- Meldonium Injection
- Digoxin Tablets
- Digoxin Tablets
- Digoxin Tablets
- Dopamine Hydrochloride Injection
- Ranolazine Tablets
- Pitavastatin Tablets
- Brain & Nervous system drugs
- Bromocriptine Mesylate Tablets
- Sodium Valproate with valproic acid Tablets
- Divalproex Sodium Extended release Tablets
- Divalproex Sodium Valproic Acid Tablets
- Sodium Valproate Tablets Bp
- Carbamazepine Tablets
- Quetiapine Tablets
- Bromocriptine Mesylate Tablets
- Cabergoline Tablets
- Ethosuximide Capsules
- Cabergoline Tablets
- Pregabalin Capsules
- Nimodipine Injections
- Mirtazapine Tablets
- Painkillers
- Amrutanjan Balm Strong
- Aceclofenac Tizanidine Tablets
- Ibuprofen Tablet
- Acetaminophen Tablets
- Diclofenac sodium Injection
- Paracetamol Tablets
- Mefenamic Acid With Dicyclomine Tablets
- Pain Killers
- Diclofenac Tablet
- Aceclofenac And Paracetamol Tablets
- Drotaverine Tablet
- Aceclofenac CR Tablets
- Diclofenac Free Acid Suspension
- Thiocolchicoside with Diclofenac sodium Capsules
- Dynapar AQ Injection
- Aceclofenac Injection
- Diclofenac Tablets
- Diclofenac sodium Capsules
- Nimesulide Tablets
- Anti Diabetic Tablet
- certification
- Contact Us
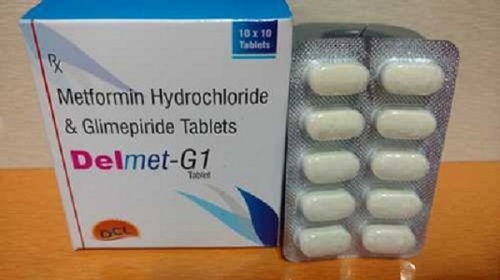
- Home
- Products
- Anti Diabetic Tablet
- Metformin Hydrochloride Sustained release and Glimepride Tablets
Metformin Hydrochloride Sustained release and Glimepride Tablets
Product Details:
- Drug Type Other Types
- Ingredients Metformin Hydrochloride 50 mg ( Sustained release ) and Glimepride 1mg Tablets
- Physical Form Tablets
- Function Other
- Dosage As directed by doctor.
- Dosage Guidelines As directed by physician.
- Suitable For Teenagers, Women, Aged Person
- Click to View more
-
Share Your Product:
Metformin Hydrochloride Sustained release and Glimepride Tablets Price And Quantity
- 100 Box
- 1 USD ($)/Box
Metformin Hydrochloride Sustained release and Glimepride Tablets Product Specifications
- Other Types
- Metformin Hydrochloride 50 mg ( Sustained release ) and Glimepride 1mg Tablets
- Teenagers, Women, Aged Person
- Other
- As directed by physician.
- Tablets
- As directed by doctor.
- Store at cool and dry place.
Metformin Hydrochloride Sustained release and Glimepride Tablets Trade Information
- Mumbai International Airport or By sea.
- Cash on Delivery (COD), Telegraphic Transfer (T/T), Western Union, Paypal, Delivery Point (DP), Letter of Credit (L/C), Letter of Credit at Sight (Sight L/C), Cash Against Delivery (CAD), Days after Acceptance (DA), Cash in Advance (CID), Cheque, Cash Advance (CA)
- 1000000 Box Per Month
- 4 Days
- Contact us for information regarding our sample policy
- 10 Tablets in a strip, 10 strips in a box with product leaflet.
- Australia, South America, Western Europe, Middle East, Central America, Asia, Eastern Europe, North America, Africa
- Dadra and Nagar Haveli, Manipur, Pondicherry, Uttarakhand, Daman and Diu, Lakshadweep, South India, North India, West India, Andaman and Nicobar Islands, Assam, Arunachal Pradesh, Chhattisgarh, Chandigarh, Goa, Jammu and Kashmir, Jharkhand, Karnataka, Kerala, Maharashtra, Mizoram, Meghalaya, Nagaland, Odisha, Punjab, Rajasthan, Sikkim, Tamil Nadu, Telangana, Tripura, West Bengal, Haryana, Delhi, Gujarat, Madhya Pradesh, Bihar, East India, Andhra Pradesh, Central India, Himachal Pradesh, Uttar Pradesh, All India
- ISO/GMP/c-GMP/WHOGMP/USFDA/EUP
Product Description
Since Last many years Saintroy Lifescience is leading manufacture, export and supplier of Metformin Hydrochloride ( Sustained release ) and Glimepiride Tablets from Surat, Gujarat, India. Metformin Hydrochloride ( Sustained release )and Glimepride Tablets used in the treatment of polycystic ovary syndrome. It is not associated with weight gain.It is taken by mouth.
Tags: Metformin Hydrochloride ( Sustained release )and Glimepride Tablets manufacturer, Metformin Hydrochloride ( Sustained release )and Glimepride Tablets exporter,Metformin Hydrochloride ( Sustained release )and Glimepride Tablets supplier Metformin Hydrochloride ( Sustained release )and Glimepride Tablets , , Glimepiride potassium, whogmp approved Glimepiride potassium tablets manufacturer and supplier, Metformin Hydrochloride ( Sustained release )and Glimepride Tablets distributor, Metformin Hydrochloride ( Sustained release )and Glimepride Tablets drop shipper, Metformin Hydrochloride ( Sustained release )and Glimepride Tablets 50 mg/ 1 mg, Metformin Hydrochloride ( Sustained release )and Glimepride Tablets 50 mg/1 mg supplier, Metformin Hydrochloride (Sustained release ) and Glimepride Tablets 50 mg /1 mg.
ISO/GMP/c-GMP/WHOGMP/NAFDAC approved, PPB Kenya approved/USFDA approved/EUGMP approved/MOH Iran approved manufacturing facility in Gujarat, India.
Registration: Saintroy Lifescience is interested in registration of own brand or customer brands in ministry of health of clients country and we also provide the products samples, COA, COPP, ACTD or CTD Dossier, DMF, Manufacturing license, Free sale certificate and other required documents.
Export Country : Saintroy Lifescience export Metformin Hydrochloride ( Sustained release ) and Glimepride Tablets to USA, Canada, Germany, Europe, France,Ghana, Nigeria, Kenya, Senegal, Sudan, UAE, Kuwait, Dubai, Cameroon, Lebanon,Peru, Chile, Somalia, Philippines, Myanmar, Maldives, Thailand, Singapore,Malaysia, Hong Kong, China, Zimbabwe, Togo, Burkina Faso, Niger, Guinea Bissau,Congo, Democratic of republic, Ivory Costa, Oman, Guatemala, Nicaragua,Salvador, Qatar, Costa Rica, Panama, Cuba, Colombia, Bolivia, Argentine,Algeria, Guyana, Morocco, Jordan, Bahrain, Iran, Egypt, Kyrgyzstan, Uzbekistan,Kazakhstan.
Dual Action for Better Diabetes Management
The combination of Metformin Hydrochloride (sustained release) and Glimepride provides a comprehensive approach to regulating blood sugar. While Metformin reduces glucose production in the liver, Glimepride increases insulin secretion from the pancreas, resulting in improved overall glycemic control.
Dosing and Administration Guidelines
Dosage must always be determined by a qualified doctor or physician based on individual health needs and response. It is vital to follow the prescribed regimen and not to self-adjust doses. The tablets are best taken with meals to minimize gastrointestinal discomfort.
Safe and Convenient Storage
To preserve the efficacy of these tablets, it is important to store them in a cool, dry place, away from excessive heat and moisture. Proper storage ensures the medicine retains its intended strength and safety until its expiry date.
FAQ's of Metformin Hydrochloride Sustained release and Glimepride Tablets:
Q: How should Metformin Hydrochloride SR and Glimepride Tablets be taken for maximum effectiveness?
A: These tablets should be taken strictly as directed by your doctor, usually with meals to reduce stomach upset. The sustained-release formulation works best when doses are consistent and not missed.Q: What are the benefits of the sustained-release formulation in these tablets?
A: The sustained-release formulation allows Metformin to be gradually released into the bloodstream, providing steady blood sugar control throughout the day and decreasing the risk of sudden spikes or drops.Q: When can I expect to see results after starting this medication?
A: Improvements in blood sugar levels are generally observed within a few days to several weeks. However, optimal glycemic control may require consistent use over an extended period, under medical supervision.Q: Where should I store these tablets to maintain their quality?
A: Store these tablets in a cool, dry place, ideally away from moisture and direct sunlight, to ensure they remain effective and safe to use until their expiration.Q: What precautions should teenagers, women, or older people take while using this medication?
A: All age groups, including teenagers, women, and seniors, should use this medication only under medical advice. Regular monitoring of blood sugar and adherence to prescribed dosages are essential for safe usage.Q: How does this medication help in managing diabetes?
A: Metformin Hydrochloride suppresses glucose production in the liver while Glimepride stimulates insulin secretion, together aiding in lowering and stabilizing blood sugar levels in individuals with type 2 diabetes.
Price:
- 50
- 100
- 200
- 250
- 500
- 1000+

Other Products in 'Anti Diabetic Tablet' category
Thank You!
Thank You for your valuable time. We have received your details and will get back to you shortly.
GST : 24DDOPK0077F1ZL
- 416, Apple Square, Nr. Swastik Plaza, Yogi Chowk, Surat - 395006, Gujarat, India
- Phone : 08071630660
- Mr Vishal N Kathiriya (Proprietor)
- Mobile :08071630660
-
 Accepts only Export inquiries
Send Inquiry
Accepts only Export inquiries
Send Inquiry
- Anti Diabetic Tablet
- Anti Fungal Tablet
- Anti Hiv Tablet
- Anti Malarial Product
- Anti Spasmodic Tablet
- Nutraceutical Product
- Pharmaceutical Syrup
- Pharmaceutical Tablets
- Pharmaceutical Capsule
- Suppositories
- Multivitamin Tablets
- Hormonal & Steroidal Products
- Antibiotic Tablets
- Immunization & Vaccination Drugs
- Antibiotic Injections
- Inhaler, Rotacaps & Respules
- Paediatric syrup & Drops
- Transdermal Patch
- Weight Loss Medicines
- Antithyroid drugs
- Antacid Products
- Pharmaceutical Infusion
- Pharmaceutical Injections
- Bulk Drugs
- Dehydrated Culture Media Supplements
- Range of DNA and Protein Tools
- Rapid Diagnostic Kit
- Medicated Soap
- Medicated Hair Oil
- Energy & Power Enhancer
- Anticancer Injection
- Anti Cancer Tablet
- Asthma & Tuberculosis Products
- Anti Allergic Tablets
- Antiviral Medicines
- Hepatitis B medicines
- Ayurvedic Product
- Herbal Tablets
- Seeds
- Pharmaceuticalcream And Gel
- Veterinary Injections
- Carbon & Chemicals
- Solvent
- Active Pharmaceutical Ingredients
- Pharmaceutical Intermediate
- Speciality Fine Chemical
- Bioreagent,Biochemicals & Speciality Fine Chemical
- Extended or Sustained Release Pellets
- Delayed Release Pellets
- Immediate Release Pellets
- Blended Release Pellets
- Eye & Ear & Nasal Drops
- Surgical Product
- Hospital & Medical Supplies
- Medical Glass Syringes
- Pharmaceutical Glass Container
- Laboratory Container
- Surgical Suture
- Medical Devices
- Nanotechnology Products
- Cardiac Product
- Brain & Nervous system drugs
- Painkillers
 |
SAINTROY LIFESCIENCE
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |